Baeyer-Villiger单加氧酶催化袋重编程用于环境相容的手性亚砜药物合成
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
质子泵抑制剂如(R)-兰索拉唑对胃病的治疗至关重要,但传统的合成依赖于对环境有害的过渡金属催化剂。在这里,我们重新编程了巴西铜(Cupriavidus basilensis, CbBVMO)的Baeyer-Villiger单加氧酶,使兰索拉唑硫合成(R)-兰索拉唑具有环境相容性。通过四氨基酸扫描策略,鉴定出来自三个残基位点的四种变体,显示出比活性增加了3倍。其中,单个突变体L315Y的比活性提高了15倍。计算研究表明,L315Y通过与R312 π -π相互作用稳定催化过渡态,导致活化能降低。随后的组合诱变得到优化的变体CbBVMOV3,其活性提高了30倍以上,达到11.6 U/mg。经过工艺优化,该变体在4 L规模的生物转化中表现出较强的催化性能,在8 h内实现了硫兰索拉唑(50 g/L) 97%的转化率。与化学方法相比,该生物催化途径将环境因子从62.6降低到4.75 kg /kg产品,生产成本降低了80%。通过消除有毒金属催化剂和减少废物产生,我们的工作进一步证明,工程BVMOs是合成手性亚砜药物的环保替代品。本文章由计算机程序翻译,如有差异,请以英文原文为准。
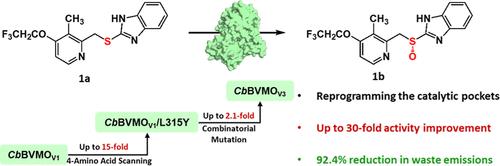
Reprogramming the Catalytic Pocket of Baeyer–Villiger Monooxygenase for Environmentally Compatible Synthesis of a Chiral Sulfoxide Pharmaceutical
Proton pump inhibitors such as (R)-lansoprazole are essential for gastric disease treatment, yet conventional syntheses rely on environmentally hazardous transition metal catalysts. Here, we reprogrammed a Baeyer–Villiger monooxygenase from Cupriavidus basilensis (CbBVMO) to enable environmentally compatible synthesis of (R)-lansoprazole from lansoprazole sulfide. By a four-amino-acid scanning strategy, a total of four variants from three residue sites exhibiting a >3-fold increase in specific activity were identified. Among them, a single mutant L315Y achieved a 15-fold increase in the specific activity. Computational studies revealed that L315Y stabilizes the catalytic transition state via π–π interactions with R312, resulting in the reduction of activation energy. Subsequent combinatorial mutagenesis yielded optimized variant CbBVMOV3 with an over 30-fold increase in activity, reaching 11.6 U/mg. Following process optimization, this variant exhibited strong catalytic performance in a 4 L-scale biotransformation, achieving 97% conversion of lansoprazole sulfide (50 g/L) within 8 h. This biocatalytic route reduces the environmental factor from 62.6 to 4.75 kgwaste/kgproduct and lowers the production cost by 80% compared to the chemical method. By eliminating toxic metal catalysts and minimizing waste generation, our work adds further evidence that engineered BVMOs are environmentally benign alternatives for synthesizing chiral sulfoxide pharmaceuticals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


