通过C-F和C-O键同时裂解,钴催化醇和三氟烯烃的交叉亲电偶联
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们报道了一种有效的交叉亲电偶联策略,用于从现成的苯醇和α-三氟甲基烯烃合成宝石二氟烯烃。这种脱氧/脱氟转化通过在温和的电化学条件下同时裂解强C-O和C-F键进行,在原位产生高活性的苄基自由基。该方法消除了多步醇预活化方案的需要,并具有广泛的底物范围和官能团耐受性。克级反应和复杂分子的后期功能化进一步证明了它的合成效用。鉴于醇的普遍存在和宝石-二氟烯烃基序在制药和材料化学中的重要性,本协议为通过自由基交叉亲电偶联形成C-C键提供了一个实用且可扩展的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
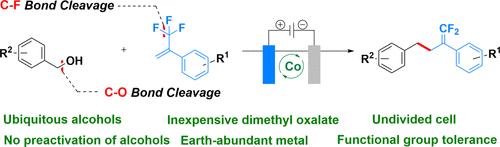
Electrochemical Cobalt-Catalyzed Cross-Electrophile Coupling of Alcohols and Trifluoroalkenes via Simultaneous C–F and C–O Bond Cleavage
We report an efficient cross-electrophile coupling strategy for the synthesis of gem-difluoroalkenes from readily available benzyl alcohols and α-trifluoromethyl alkenes. This deoxygenative/defluorinative transformation proceeds via the simultaneous cleavage of strong C–O and C–F bonds under mild electrochemical conditions, generating highly reactive benzyl radicals in situ. The methodology eliminates the need for multistep alcohol preactivation protocols and exhibits a broad substrate scope with functional group tolerance. Its synthetic utility is further demonstrated by gram-scale reactions and the late-stage functionalization of complex molecules. Given the ubiquity of alcohols and the importance of gem-difluoroalkene motifs in pharmaceutical and materials chemistry, this protocol offers a practical and scalable platform for C–C bond formation via radical cross-electrophile coupling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


