二氧化硅负载氧化锌催化剂催化乙醇脱氢制乙醛的构效关系
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
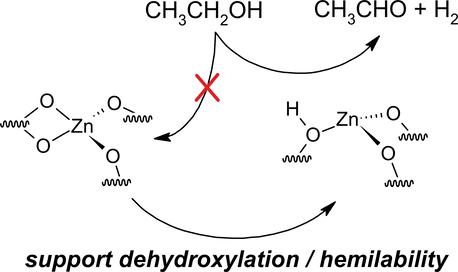
二氧化硅负载ZnO有效催化乙醇非氧化脱氢制乙醛,这与生物乙醇生产1,3-丁二烯有关。脱水条件下的原位光谱(高灵敏度-低能离子散射(HS-LEIS)、漫反射(DR) UV - vis、x射线吸收光谱(XAS)、漫反射傅立叶变换红外光谱(DRIFTS)、非弹性中子散射(INS)和紫外拉曼光谱)和氨吸附(通过程序升温解吸,然后进行DRIFTS和质谱(DRIFTS- ms NH3-TPD))进行表征。DFT计算表明,负载型ZnOx相以孤立的表面ZnOx位点存在于SiO2上,绝大多数由两个硅氧烷键和一个硅原子与两个非桥接氧((≡SiO)2Zn2+O2Si=)配位,锚定在4元、5元和6元硅氧烷环上。少量的表面ZnOx位点具有Lewis酸性,更少的位点具有Bro -嵌套酸性Zn(OH)+Si基团。乙醇温控表面反应质谱(TPSR-MS)与各种氧化或乙醇反应预处理表明,只有具有Lewis和Bro系列酸性特征的位点(Zn(OH)+Si)对乙醇脱氢有活性,而大多数表面(≡SiO)2Zn2+O2Si=位点不具有活性。通过原位DR紫外-可见光谱评估,所有表面ZnOx位点之间的异质性较大,与乙醇脱氢活性的ZnOx位点数量较多以及在最活跃的表面ZnOx位点中乙醛生产的焓垒较低有关。用稳态动力学方法测定了乙醇脱氢的周转频率和表观活化能。综上所述,这些发现表明,在二氧化硅载体上锚定非活性表面(≡SiO)2Zn2+O2Si=位点会导致更多的活性表面ZnOx位点采用更应变的构型,从而促进乙醇脱氢催化。在TPSR过程中,促进乙醇解吸的预处理和催化剂(作为表面去羟基化的标志)与最活跃的表面(Zn(OH)+Si)位点的数量增加有关。这些发现表明,通过载体的去羟基化和/或减少与半不稳定硅氧烷配体的配位,活性表面ZnOx位点被激活以进行乙醇脱氢。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structure–Activity Relationships for Ethanol Dehydrogenation to Acetaldehyde by Silica-Supported Zinc Oxide Catalysts
Silica-supported ZnO efficiently catalyzes the nonoxidative dehydrogenation of ethanol to acetaldehyde, which is relevant for production of 1,3-butadiene from bioethanol. Characterization with in situ spectroscopies under dehydrated conditions (high sensitivity-low energy ion scattering (HS-LEIS), diffuse reflectance (DR) UV–vis, X-ray absorption spectroscopy (XAS), diffuse reflectance Fourier transform infrared spectroscopy (DRIFTS), inelastic neutron scattering (INS), and UV Raman), and ammonia adsorption probed by temperature-programmed desorption followed by DRIFTS and mass spectrometry (DRIFTS-MS NH3-TPD), and DFT calculations revealed that the supported ZnOx phase was present as isolated surface ZnOx sites on SiO2, with the vast majority coordinated by two siloxane bonds and one silicon atom with two nonbridging oxygens ((≡SiO)2Zn2+O2Si=), anchored at 4-, 5-, and 6-membered siloxane rings. A minor fraction of surface ZnOx sites possessed Lewis acidity, and even fewer sites possessed a Bro̷nsted acidic Zn(OH)+Si moiety. Ethanol temperature-programmed surface reaction-mass spectrometry (TPSR-MS) with various oxidative or ethanol reaction pretreatments indicated that only sites with Lewis and Bro̷nsted acidic character (Zn(OH)+Si) were active for ethanol dehydrogenation, while the majority surface (≡SiO)2Zn2+O2Si= sites were inactive. Greater heterogeneity among all surface ZnOx sites, as assessed by in situ DR UV–vis spectroscopy, was associated with a greater number of ZnOx sites that were active for ethanol dehydrogenation as well as lower enthalpic barriers for acetaldehyde production among the most active surface ZnOx sites. Turnover frequencies and the apparent activation energy for ethanol dehydrogenation were determined from steady-state kinetics. Together, these findings suggested that anchoring inactive surface (≡SiO)2Zn2+O2Si= sites on the silica support caused a greater number of active surface ZnOx sites to adopt a more strained configuration, promoting ethanol dehydrogenation catalysis. Pretreatments and catalysts that promoted desorption of ethanol during TPSR, taken as a marker of surface dehydroxylation, were associated with an increased number of the most active surface (Zn(OH)+Si) sites. Such findings suggested that inactive surface ZnOx sites were activated for ethanol dehydrogenation by dehydroxylation of the support and/or decreased coordination to hemilabile siloxane ligands.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


