CuO@Fe2O3对过氧单硫酸盐的协同活化增强氧氟沙星的降解:表面羟基的作用和环境适用性
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
研究了一种Cu/Fe双金属材料,用于过氧单硫酸盐(PMS)活化降解有机污染物。在相同条件下,双金属材料CuO@Fe2O3对氧氟沙星(OFX)的降解动力学常数分别比单金属CuO和Fe2O3催化剂高2.15倍和28.19倍。铜和铁的协同作用促进了催化反应的发生,提高了降解效率。通过自由基猝灭实验、氧化氘(D2O)实验、预混合实验和自由基贡献率计算,证实了该反应涉及自由基和非自由基途径的协同作用。在碱性条件下,产生表面羟基,加速了反应过程,在5分钟内达到近100%的去除率。通过LC-MS鉴定中间体并结合理论计算,提出了OFX的潜在降解途径。降解副产物的低毒性和材料强大的抗干扰能力、可重复使用性和结构稳定性共同证明了其在水修复应用中的实际潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
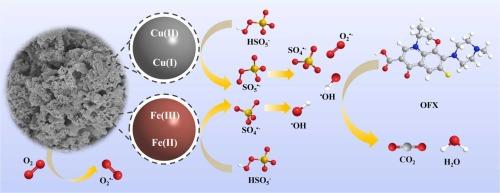
Synergistic activation of peroxymonosulfate by CuO@Fe2O3 for enhanced degradation of ofloxacin: Role of surface hydroxyl groups and environmental applicability
This study develops a Cu/Fe bimetallic material for peroxymonosulfate (PMS) activation to degrade organic pollutants. Under identical conditions, the degradation kinetic constant of ofloxacin (OFX) by the bimetallic material CuO@Fe2O3 was 2.15-fold and 28.19-fold higher than those achieved with single-metal CuO and Fe2O3 catalysts, respectively. The synergistic effect of copper and iron species promoted the occurrence of catalytic reaction and improved the degradation efficiency. Through radical quenching experiments, deuterium oxide (D2O) experiments, pre-mixing experiments, and calculations of radical contribution rates, it was confirmed that the reaction involves the synergistic action of both free radical and non-free radical pathways. Under alkaline conditions, surface hydroxyl groups are generated, which accelerate the reaction process, achieving a nearly 100% removal rate within 5 minutes. Potential degradation pathways of OFX were proposed through LC-MS identification of intermediates coupled with theoretical calculations. The low toxicity of degradation byproducts and the material's robust anti-interference capability, reusability, and structural stability collectively demonstrate its practical potential for water remediation applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


