基因工程糖基磷脂酰肌醇锚定抗gd2纳米体外泌体模拟物用于骨肉瘤的体外和体内靶向治疗
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
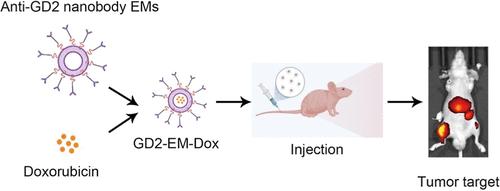
本研究的重点是外泌体模拟物(EMs)的工程设计,该外泌体被抗双胞脂苷(GD2)纳米体修饰,用于靶向骨肉瘤。我们将抗gd2纳米体编码载体导入HEK-293T (293T)细胞,并将其与衰变加速因子(DAF)衍生的糖基磷脂酰肌醇(GPI)锚定信号肽融合。采用序贯挤压法制备目标材料。这些工程化的gd2靶向emm (GD2-EMs)具有与常规外泌体相同的外泌体标记,大量生产,具有纳米级尺寸,并具有表面抗gd2纳米体的特征。此外,与未修饰的EMs相比,它们在骨肉瘤细胞中的内化程度增加。在裸鼠动物实验中,GD2-EMs表现出优越的肿瘤靶向性,证实了其在体内的有效性。总之,该研究成功地设计了抗gd2 emms,作为骨肉瘤的药物递送系统显示出有希望的结果,表明工程抗gd2 emms可能是对抗骨肉瘤的可行方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Genetically Engineered Glycosylphosphatidylinositol-Anchored Anti-GD2 Nanobody-Exosome Mimetics for Targeted Osteosarcoma Therapy In Vitro and In Vivo
This study focuses on the engineering of exosome mimetics (EMs) that are decorated with an antidisialoganglioside (GD2) nanobody for targeting osteosarcoma. We engineer the anti-GD2 nanobody coding vector into HEK-293T (293T) cells and fuse it with the decay-accelerating factor (DAF)-derived glycosylphosphatidylinositol (GPI) anchor signal peptide. The sequential extrusion method was used to produce the targeted EMs. These engineered GD2-targeted EMs (GD2-EMs) possess the same exosome markers as regular exosomes, are produced in large quantities, have a nanoscale size, and feature a surface anti-GD2 nanobody. Furthermore, they demonstrate increased internalization in osteosarcoma cells compared to unmodified EMs. In animal experiments using nude mice, the GD2-EMs exhibited superior tumor targeting, confirming their efficacy in vivo. In conclusion, the study successfully engineered anti-GD2 EMs, which showed promising results as a drug delivery system for osteosarcoma, indicating that engineering anti-GD2 EMs may be a viable approach for combating this disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


