基于分子印迹聚吡咯的电化学水杨酸传感器
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究旨在为基于分子印迹聚合物(MIP)的电化学水杨酸(SA)传感器的开发提供新的见解。将聚吡咯(Ppy)基MIP层和非印迹聚合物(NIP)层沉积在铂电极上,并在三电极电化学电池中进行了表征。该研究使用安培法进行单体聚合,循环伏安法(CV)用于聚合物层的过氧化,差分脉冲伏安法(DPV)用于分析物检测。通过比较SA与3-羟基苯甲酸和三聚氰胺的电化学信号来评价其选择性。结果证实了电化学传感器的选择性。利用密度泛函理论(DFT)分析了重结合和识别机制。DFT计算结果与实验结果一致。总之,与三聚氰胺及其异构体3-羟基苯甲酸(3-HBA)相比,基于聚吡咯的MIP传感器对水杨酸的检测具有更高的选择性和灵敏度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
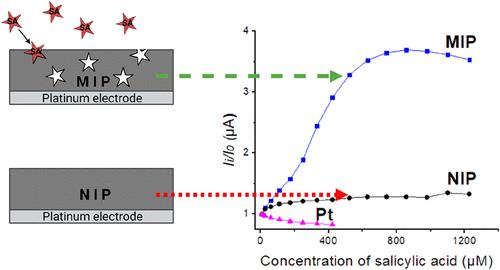
Electrochemical Salicylic Acid Sensor Based on Molecularly Imprinted Polypyrrole
This study aims to provide new insights into the development of an electrochemical salicylic acid (SA) sensor based on a molecularly imprinted polymer (MIP). Polypyrrole (Ppy) based MIP and nonimprinted polymer (NIP) layers were deposited on the platinum electrode and evaluated in a three-electrode electrochemical cell. The study used amperometry for monomer polymerization, cyclic voltammetry (CV) for the overoxidation of the polymer layer, and differential pulse voltammetry (DPV) for analyte detection. Selectivity was evaluated by comparing the electrochemical signals of SA with those of 3-hydroxybenzoic acid and melamine. Results confirm the selectivity of the electrochemical sensor. Density functional theory (DFT) calculations were performed to analyze the rebinding and recognition mechanism. The results of DFT calculations support the experimental findings. In conclusion, the polypyrrole-based MIP sensor exhibits higher selectivity and sensitivity toward salicylic acid detection compared to melamine and even to its isomer, 3-hydroxybenzoic acid (3-HBA).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


