海洋沉积物中纳米晶氟磷灰石中钇的结构形式(0.11 Å分辨率)
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
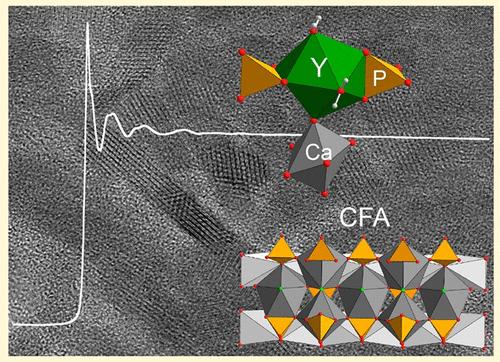
深海泥中稀土元素和钇(REY)含量丰富,其中钇(Y)含量最高。REY存在于自生(a-CFA)和生物源(b-CFA)碳酸盐氟磷灰石(CFA, Ca5(CO3)x(PO4) 3-xF1 +x)中。REY在两种CFA类型中的存在表明在深海环境中的富集过程不同,这可以通过REY配位化学的详细结构分析来追踪。利用扩展x射线吸收精细结构(EXAFS)光谱在2018年和2023年以0.15 Å的分辨率研究了Y在CFA中的键合环境。虽然这些研究为Y的短期秩序提供了有价值的见解,但它们也存在不一致性。此外,0.15 Å的分辨率不足以揭示CFA中Y的复杂局部结构。在这里,我们展示了在太平洋海底几米以下收集的a- cfa和b-CFA中Y的分辨率为0.11 Å的EXAFS数据。Y在a-CFA和b-CFA纳米晶体周围以非晶相的形式与Ca和PO4结合,并二次结合到a-CFA的晶体结构中。没有EXAFS证据表明存在多核Y沉淀,这与最近在铈(Ce)上的发现相反,也不支持Y-碳酸盐络合物的形成。后两个发现得到了密度泛函理论的支持,这表明Y-Y对的形成在热力学上是不利的,并且预测的Y-C距离与EXAFS距离不一致。本研究强调了深海沉积物中Y的地球化学富集,通过在a- cfa和b-CFA中形成水合钇-磷酸钙相,以及在钙和磷酸盐共沉淀过程中Y在自生a- cfa纳米晶体中取代Ca。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structural Form of Yttrium in Nanocrystalline Fluorapatite from Marine Sediments at 0.11 Å Resolution
Deep-sea mud is rich in rare earth elements and yttrium (REY), with yttrium (Y) exhibiting the highest concentration. REY are found in authigenic (a-CFA) and biogenic (b-CFA) carbonate fluorapatite (CFA, Ca5(CO3)x(PO4)3–xF1+x). The presence of REY in both CFA types suggests different enrichment processes in abyssal environments, which may be traced through detailed structural analysis of REY’s coordination chemistry. The bonding environment of Y in CFA was investigated in 2018 and 2023 using extended X-ray absorption fine structure (EXAFS) spectroscopy at a resolution of 0.15 Å. While these studies offered valuable insights into Y’s short-range order, they also presented inconsistencies. Moreover, a resolution of 0.15 Å is insufficient to uncover the intricate local structure of Y in CFA. Here, we present EXAFS data at a resolution of 0.11 Å for Y in a-CFA and b-CFA collected several meters beneath the Pacific Ocean seafloor. Y is predominantly hydrated and bound to Ca and PO4 in an amorphous phase surrounding the a-CFA and b-CFA nanocrystals and is secondarily incorporated into the crystal structure of a-CFA. There is no EXAFS evidence indicating the presence of polynuclear Y precipitate, which contrasts with a recent finding on cerium (Ce), nor supporting the formation of a Y-carbonate complex. The latter two findings are backed by density functional theory, which indicates that Y–Y pair formation is thermodynamically unfavorable and that the predicted Y–C distance is inconsistent with the EXAFS distances. This research highlights the geochemical enrichment of Y in abyssal sediments through the formation of a hydrated yttrium–calcium phosphate phase in a-CFA and b-CFA and Y for Ca substitution in authigenic a-CFA nanocrystals during the coprecipitation of calcium and phosphate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


