重塑二价染色质是小鼠着床期胚胎发生的必要条件。
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
二价在谱系承诺过程中调控发育基因。然而,二价结构域在早期胚胎发生中建立、维持和解决的机制尚不清楚。在这里,我们全面追踪了小鼠着床周围发育过程中的二价染色质重塑,揭示了外胚层和原始内胚层调控程序的分裂建立模式。我们在外胚层中发现了短暂维持的二价结构域(TB结构域),其中逐渐的分解微调了多能性的进展。通过在胚胎中进行靶向筛选,我们发现了22个TB结构域调节因子,包括必需因子ZBTB17。基因消融或ZBTB17的降解导致着床期骤停。在机制上,ZBTB17与KDM6A/B合作,通过去除H3K27me3和启动关键多能性基因的激活来解决二价性问题。值得注意的是,在人类多能性转变过程中,TB结构域动力学在进化上是共享的,尽管物种存在差异,但ZBTB17也参与其中。我们的工作建立了早期哺乳动物发育中二价染色质调控的框架,并阐明了其分辨率如何精确控制谱系承诺。本文章由计算机程序翻译,如有差异,请以英文原文为准。
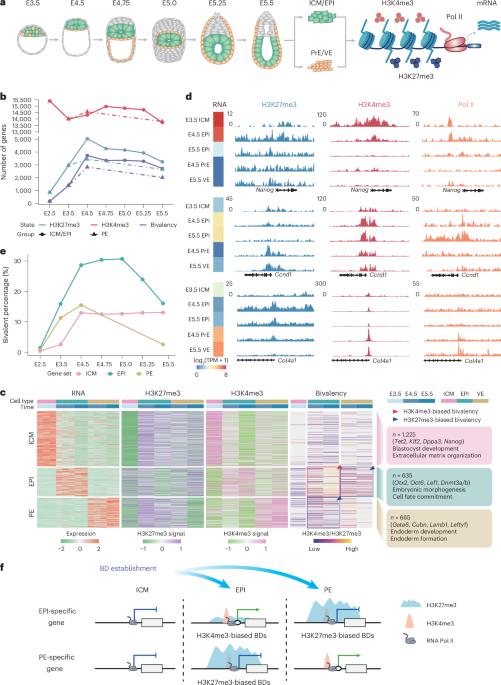
Remodelling bivalent chromatin is essential for mouse peri-implantation embryogenesis
Bivalency regulates developmental genes during lineage commitment. However, mechanisms governing bivalent domain establishment, maintenance and resolution in early embryogenesis remain unclear. Here we comprehensively trace bivalent chromatin remodelling throughout mouse peri-implantation development, revealing bifurcated establishment modes that partition epiblast and primitive endoderm regulatory programmes. We identify transiently maintained bivalent domains (TB domains) enriched in the epiblast, where gradual resolution fine-tunes pluripotency progression. Through targeted screening in embryos, we uncover 22 TB domain regulators, including the essential factor ZBTB17. Genetic ablation or degradation of ZBTB17 causes peri-implantation arrest. Mechanistically, ZBTB17 collaborates with KDM6A/B to resolve bivalency by removing H3K27me3 and priming the activation of key pluripotency genes. Remarkably, TB domain dynamics are evolutionarily shared in human pluripotent transitions, with ZBTB17 involvement despite species differences. Our work establishes a framework for bivalent chromatin regulation in early mammalian development and elucidates how its resolution precisely controls lineage commitment. Li, He, Liu and colleagues characterize the dynamic bivalent chromatin landscape during mouse peri-implantation development. They find that factor ZBTB17 works with KDM6A/B to resolve transiently maintained bivalent domains and prime gene activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


