对耐甲氧西林金黄色葡萄球菌(MRSA)具有明显抗菌活性的强效ClpX调节剂的发现
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
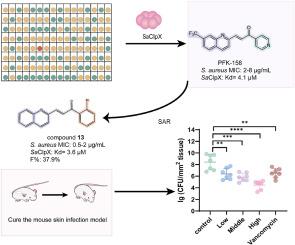
侵袭性耐甲氧西林金黄色葡萄球菌(MRSA)感染的高发病率和死亡率强调了开发新型抗生素的迫切需要。ClpX作为ClpP的一种协伴侣蛋白,已被确定为对抗MRSA的重要靶点。在这项研究中,我们筛选了一个内部文库,并鉴定了一个小分子化合物PFK-158,对金黄色葡萄球菌ClpX具有高亲和力(Kd = 4.1 μM)。同时,PFK-158对MRSA表现出较强的抗MRSA活性(MIC = 2 μg/mL, MBC = 8 μg/mL)。进一步优化得到一种新的α, β-不饱和酮,含有喹啉基和2-溴苯基取代基,与金黄色葡萄球菌ClpX具有相当的结合亲和力(Kd = 3.6 μM),对MRSA具有较强的抗菌活性,诱导耐药倾向较低。值得注意的是,具有喹啉基和2-溴苯基取代基的新型α, β-不饱和酮具有良好的体内安全性,口服生物利用度(F = 37.9 %),并且在mrsa感染的皮肤脓肿模型中具有良好的治疗效果。总的来说,我们的研究结果提出了一种具有抗葡萄球菌感染潜力的新型SaClpX调节剂,并为进一步开发特异性金黄色葡萄球菌ClpX调节剂提供了一个有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery of potent ClpX modulators with pronounced antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA)
The high morbidity and mortality rates associated with invasive methicillin-resistant Staphylococcus aureus (MRSA) infections underscore the pressing need to develop novel antibiotics. ClpX, functioning as a cochaperone protein of ClpP, has been identified as a crucial target in combating MRSA. In this study, we screened an in-house library and identified a small molecule compound, PFK-158, with high affinity for Staphylococcus aureus ClpX (Kd = 4.1 μM). Simultaneously, PFK-158 displayed potent activity against MRSA (MIC = 2 μg/mL, MBC = 8 μg/mL). Further optimization resulted in a novel α, β-unsaturated ketone bearing a quinolinyl group and a 2-bromophenyl substituent with comparable binding affinity for Staphylococcus aureus ClpX (Kd = 3.6 μM), and it showed enhanced antibacterial activity against MRSA and lower propensity for inducing resistance. Significantly, the novel α, β-unsaturated ketone bearing a quinolinyl group and a 2-bromophenyl substituent demonstrated favorable in vivo safety, oral bioavailability (F = 37.9 %), and promising therapeutic effects in a MRSA-infected skin abscess model. Overall, our findings presented a novel SaClpX modulator with the potential to combat Staphylococcus infections, and suggested a promising strategy for the further development of specific Staphylococcus aureus ClpX modulators.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


