通过人体血浆样品的快速声学分离来获取细胞外囊泡的蛋白质组
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
尽管质谱技术取得了长足的进步,但在测定血浆中细胞外囊泡(EVs)蛋白质组方面仍然存在障碍。最近的工作表明,分离ev可以使检测血浆中丰度较低的蛋白质成为可能。常用的EV分离方法要么需要大样本量和较长的超离心时间,要么通过靶向分离导致群体偏倚。需要一种快速简便的方法从10 μL的小体积血浆中分离出ev,从而能够发现生物标志物,例如在生物银行样品中,质谱法可以发挥重要作用。结果利用种子粒子增强声捕获技术,在6分钟内从8 μL的微量血浆样品中纯化ev,揭示了细胞外囊泡蛋白质组。差分质谱分析结果发现,与原始(未处理)血浆相比,声学捕获样品中的蛋白质显著富集(经fdr调整的p值0.05),其中超过三分之二的蛋白质先前与ev相关。此外,通过检测51种未在原始血浆中检测到的低丰度蛋白质,我们能够增加分析的深度,其中一半被标记为基因本体(GO)标签“细胞外泌体”(GO:0070062)。最后,我们验证了中性带电二氧化硅种子颗粒与200 μL/min洗涤流速配对的新方法,使处理时间缩短了一半,并发现了与30 μL/min洗涤的聚苯乙烯种子颗粒相同的蛋白质组。我们基于微流体技术的EV分离方法能够快速处理单个微小血浆样本,表明当使用声捕获作为预处理步骤时,可以检测到与EV相关的个人蛋白质组学信息。通过将该技术应用于患者队列或小鼠模型的血浆,未来的研究可能会为ev在疾病进展中的作用提供新的见解,并揭示ev蛋白质组学货物中的新的诊断靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
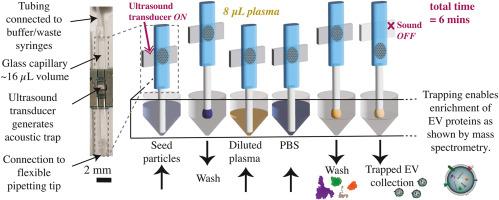
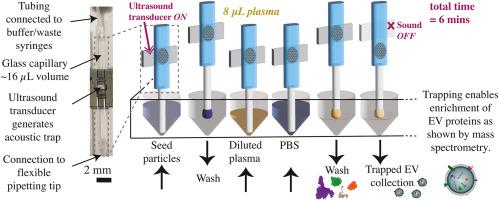
Accessing the proteome of extracellular vesicles via rapid acoustic isolation of a minute human blood plasma sample
Background
Despite substantial progress in the field of mass spectrometry, there remain barriers to measuring the extracellular vesicles (EVs) proteome in blood plasma. Recent work has shown that isolating EVs can make it possible to detect proteins that have low abundance in plasma. Commonly used EV isolation methods either require large sample volumes and long ultracentrifugation times, or else result in population bias via targeted isolation. There is a great need for fast and easy methods to isolate EVs from small volumes of plasma, <10 μL, enabling biomarker discovery, e.g. in biobanked samples, where mass spectrometry can play an important role.
Results
We unveil the extracellular vesicle proteome by using seed particle enhanced acoustic trapping to purify EVs from minute blood plasma samples (8 μL) in 6 min per sample. The differential mass spectrometry results find proteins which are significantly enriched (FDR-adjusted p-values<0.05) in acoustically trapped samples compared to raw (unprocessed) plasma, more than two thirds of those proteins have been associated with EVs previously. Additionally, we are able to increase the depth of analysis by detecting 51 low abundance proteins not detected in raw plasma, half of which are tagged with the gene ontology (GO) tag “extracellular exosome” (GO:0070062). Finally, we validate the novel use of neutrally charged silica seed particles paired with a washing flowrate of 200 μL/min, enabling the processing time to be halved and finding the same proteome as for tried-and-tested polystyrene seed particles with washing at 30 μL/min.
Significance
Our microfluidics-based approach to EV isolation enables rapid processing of an individual minute blood plasma sample, demonstrating that personal proteomic information associated with EVs can be detected when acoustic trapping is used as a pre-processing step. By applying this technique to plasma from patient cohorts or mouse models, future studies may offer new insights into the role of EVs in the progression of diseases and reveal new diagnostic targets in the proteomic cargo of EVs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


