MALDI质谱成像在健康对照和透明细胞肾细胞癌组织中与免疫检查点抑制反应相关的n -糖基化的空间图谱
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
透明细胞肾细胞癌(ccRCC)是肾癌中最常见的亚型。虽然大多数病例可以早期发现并通过手术治疗,但有一定比例的患者出现转移并需要药物治疗。免疫检查点抑制(ICI)已成为转移性ccRCC患者治疗的关键组成部分,并可能在一小部分患者中产生完全缓解。目前,还没有预测ICI反应的生物标志物,可以指导个性化治疗,提高缓解率,并最大限度地减少不太可能从ICI获益的患者的不必要治疗。结果在本研究中,我们采用基质辅助激光解吸/电离质谱成像技术来表征健康对照肾脏和21例治疗naïve原发性RCC肿瘤样本的n -糖。患者在肿瘤切除后接受ICI治疗,并根据疗效进行分层。两种候选n -聚糖在无进展生存期较长且对PD-1/PD-L1检查点抑制有反应的组中被发现上调。此外,我们建立了n -糖基化模式,对应于肾脏微观解剖结构,包括近端曲小管,远端曲小管和肾小球。为了更全面地评估不同免疫治疗反应组的肿瘤微环境,我们还进行了创新的免疫组织化学分析,通过质谱可视化。意义:本研究扩展了正常肾n -糖的空间特征。此外,比较了ccRCC组织相对于正常组织的n -糖苷,以及对ICI的反应。候选的基于聚糖的生物标志物最终可以开发用于临床转化应用的基线,以预测ICI的应答者和无应答者。本文章由计算机程序翻译,如有差异,请以英文原文为准。
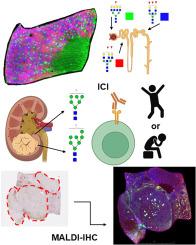
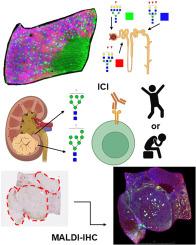
Spatial atlasing of N-glycosylation in healthy control and clear cell renal cell carcinoma tissues linked with immune checkpoint inhibition response by MALDI mass spectrometry imaging
Background
Clear cell renal cell carcinoma (ccRCC) is the most common subtype of kidney cancer. While most cases are detected early and treated by surgery, a relevant proportion of patients present with metastases and require medical treatment. Immune checkpoint inhibition (ICI) has become a key component of treatment for metastatic ccRCC patients and may produce complete responses in a fraction of patients. Presently, there is no predictive biomarker for response to ICI which could guide individualized treatments, enrich response rates and minimize unnecessary treatment in patients unlikely to benefit from ICI.
Results
In this study, we employed Matrix Assisted Laser Desorption/Ionization Mass Spectrometry Imaging to characterize the N-glycome of healthy control kidneys and 21 treatment naïve, primary RCC tumor samples. Patients were treated with ICI following tumor resection and stratified by response. Two candidate N-glycans were found to be upregulated in the group that experienced longer progression free survival and exhibited a response to PD-1/PD-L1 checkpoint inhibition. Additionally, we established N-glycosylation patterns which correspond to renal microanatomic structures including proximal convoluted tubules, distal convoluted tubules, and glomeruli. To more fully evaluate the tumor microenvironment of the different immunotherapy responder groups, we also conducted innovative immunohistochemical analyses visualized via mass spectrometry.
Significance
This study represents an expanded spatial characterization of the normal renal N-glycome. Additionally, the N-glycomes of ccRCC tissues were compared relative to normal tissues, and in context of response to ICI. Candidate glycan-based biomarkers could eventually be developed for clinical translational applications at baseline to predictively identify responders and non-responders to ICI.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


