含Sb(V)黄铁矾转化过程中锑的迁移率:去铁胺B和草酸的影响
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
锑的毒性和致癌性使其污染成为一个不容忽视的问题。在矿区,黄铁矾是Sb(V)的主要寄主矿物,其转化可能会严重影响Sb的命运。本研究考察了Sb(V)共沉淀黄铁矾在草酸(OA)和地铁胺B (DFOB)存在下的转化,并探讨了Sb的重分配。结果表明,OA和DFOB延缓黄铁矾的溶解,但促进Fe(III)和Sb(V)的释放,起到了共同的延缓和促进作用。在不使用OA或DFOB处理时,黄铁矾转化为赤铁矿和针铁矿。在仅含DFOB的体系中,DFOB没有改变黄钾铁矾的转化途径;主要产物仍为针铁矿和赤铁矿,形成Fe(III)-DFOB水络合物。在纯oa体系中,水中Fe(III)-OA配合物在矿物表面的再吸附可能导致另一种再结晶途径,形成针铁矿。在OA-DFOB体系中,DFOB与OA竞争Fe(III),形成Fe(III)-DFOB配合物,延迟OA诱导的黄铁矾向针铁矿的转变。所有反应结束时,新形成的次生Fe(III)矿物保留了总Sb的99.94%以上。OA使更多的Sb变为可磷酸盐提取的Sb, DFOB使以上可磷酸盐提取的Sb变为结晶度较差的相或残留相。本文章由计算机程序翻译,如有差异,请以英文原文为准。
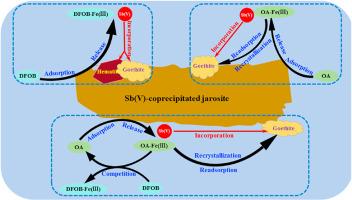
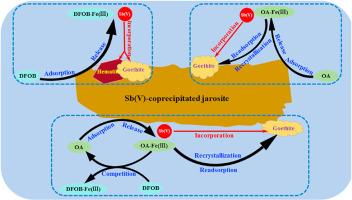
Mobility of antimony during Sb(V)-bearing jarosite transformation: Influence of desferrioxamine B and oxalic acid
Antimony (Sb) pollution is becoming a non-negligible issue due to its toxic and carcinogenic properties. In mining areas, jarosite is a main host mineral for Sb(V), and its transformation may seriously influence the fate of Sb. In this study, we examined the transformation of Sb(V)-coprecipitated jarosite in the presence of oxalic acid (OA) and Desferrioxamine B (DFOB), and explored the resulting repartition of Sb. Results showed that OA and DFOB retarded jarosite dissolution but promoted Fe(III) and Sb(V) release, playing a combined role in this retardation and promotion. In treatment without OA or DFOB, jarosite transformed into hematite and goethite. In DFOB-only systems, DFOB did not change the jarosite transformation pathway; goethite and hematite were still the dominant products, and aqueous Fe(III)-DFOB complexes were formed. In OA-only systems, the re-adsorption of aqueous Fe(III)-OA complexes on the mineral surface may result in another recrystallization pathway, forming goethite. In OA-DFOB systems, DFOB competed with OA for Fe(III), forming Fe(III)-DFOB complexes and delayed OA-induced jarosite transformation into goethite. By the end of all reactions, more than 99.94 % of total Sb was retained by neo-formed secondary Fe(III) minerals. OA drove more Sb to become phosphate-extractable, and DFOB drove the above phosphate-extractable Sb into poorly crystalline phases or residual phases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


