20年的闪蒸纳米沉淀——从可控沉淀到全球医学
IF 17.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
Flash nanop沉淀法(FNP)技术是一种使用快速湍流混合来驱动聚合物或脂质纳米颗粒自组装的抗溶剂沉淀技术,自开发以来的二十年中,该平台已被用于研究和工业中的各种药物输送应用-最值得注意的是作为全球生产的辉瑞- biontech COMIRNATY®mRNA脂质纳米颗粒疫苗的使能技术。重要的是,这使得FNP成为全球商业规模的脂质纳米颗粒配方中唯一公开的制造技术。这种情况使得该技术引人注目,值得广泛讨论,这也是本文所要做的。它还将FNP混合作为基准技术,与其他LNP制造工艺进行比较。本文综述了这种连续抗溶剂沉淀技术的原理、其可扩展性和在下游单元操作中的应用,以及它在纳米医学研究中的应用。我们讨论了围绕FNP技术的当前知识产权格局,并举例说明了其在SARS-CoV-2和低成本抗疟制剂中的工业实施。最后,我们对平台的最新改进和扩展进行了调查,这些改进和扩展使FNP进入第三个十年时能够封装新类别的分子,并在制造中具有更大的灵活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
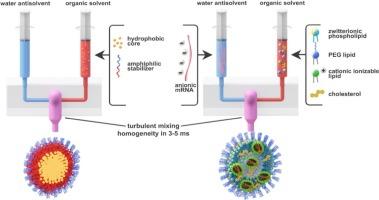
20 years of flash nanoprecipitation – from controlled precipitation to global medicine
In the twenty years since the development of Flash NanoPrecipitation (FNP) technology, an antisolvent precipitation technique that uses rapid turbulent mixing to drive self-assembly of polymeric or lipid nanoparticles, the platform has been used for a wide variety of drug delivery applications in research and industry – most notably as the enabling technology for the global manufacture of the Pfizer-BioNTech COMIRNATY® mRNA lipid nanoparticle vaccine against SARS-CoV-2. Importantly, this makes FNP the only publicly-known manufacturing technology for global commercial-scale lipid nanoparticle formulation. This situation makes the technique remarkable and noteworthy and worth discussing broadly, which this article aims to do. It also sets FNP mixing as the benchmark technology against which other LNP manufacturing processes should be compared. Here we review the principles underpinning this continuous antisolvent precipitation technique, its scalability and use with downstream unit operations, and its utility in nanomedicine research. We discuss the current intellectual property landscape surrounding FNP technology and give examples of its industrial implementation for SARS-CoV-2 and low-cost antimalarial formulations. We end with a survey on recent improvements and extensions to the platform that enable the encapsulation of new classes of molecules and greater flexibility in manufacturing as FNP moves into its third decade.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
28.10
自引率
5.00%
发文量
294
审稿时长
15.1 weeks
期刊介绍:
The aim of the Journal is to provide a forum for the critical analysis of advanced drug and gene delivery systems and their applications in human and veterinary medicine. The Journal has a broad scope, covering the key issues for effective drug and gene delivery, from administration to site-specific delivery.
In general, the Journal publishes review articles in a Theme Issue format. Each Theme Issue provides a comprehensive and critical examination of current and emerging research on the design and development of advanced drug and gene delivery systems and their application to experimental and clinical therapeutics. The goal is to illustrate the pivotal role of a multidisciplinary approach to modern drug delivery, encompassing the application of sound biological and physicochemical principles to the engineering of drug delivery systems to meet the therapeutic need at hand. Importantly the Editorial Team of ADDR asks that the authors effectively window the extensive volume of literature, pick the important contributions and explain their importance, produce a forward looking identification of the challenges facing the field and produce a Conclusions section with expert recommendations to address the issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


