多界面微泡控制协同血管破坏/化疗治疗的顺序空化
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
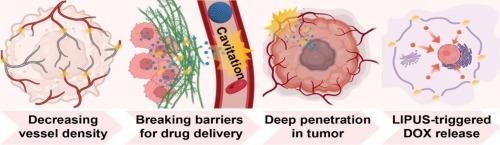
微泡已成为生物医学中多功能的治疗平台。除了用作超声造影剂,利用空化介导的物理效应,微泡现在可以实现靶向药物输送和精确肿瘤消融。在本研究中,我们通过疏水介孔二氧化硅纳米颗粒(hMSNs)的界面自组装来设计负载阿霉素(DOX)的多界面微泡(DOX-MIMBs),建立了具有持续声活性的分层结构的MIMBs。hmsn与气液界面的亲和力强,有利于空化效应的传递。在低强度超声(<3 W/cm2)照射下,初级MIMBs坍塌产生次级子泡,这些子泡通过hmsn介导的气液界面重建迅速稳定,并能够再次空化。这一过程实现了能量级联空化——连续几代气泡持续存在,直到声波能量耗散,与传统的脂质壳微气泡相比,实现了更长的空化持续时间。DOX- mimbs产生的顺序声力学扰动诱导了协同肿瘤治疗:选择性血管破坏机械塌陷的未成熟肿瘤血管和增强化疗,使DOX在肿瘤中分布更广、渗透更深。DOX- mimbs利用序贯泡空化诱导冲击波和微流,结合肿瘤血管机械破坏和肿瘤深部DOX穿透化疗,在肾细胞癌模型中实现肿瘤体积适当缩小90 %。这种精心设计的DOX-MIMBs机械药物传递系统实现了从全身药物轰击到局部机械化学肿瘤抑制的范式转变,为肿瘤精准治疗提供了强有力的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Multi-interfacial microbubbles controlled sequential cavitation for synergistic vascular destruction/chemotherapeutic therapy
Microbubbles have emerged as versatile theranostic platforms in biomedicine. In addition to being used as ultrasound contrast agents, capitalizing on cavitation-mediated physical effects, microbubbles now enable targeted drug delivery and precision tumor ablation. In this study, we engineer doxorubicin (DOX)-loaded multi-interfacial microbubbles (DOX-MIMBs) through interfacial self-assembly of hydrophobic mesoporous silica nanoparticles (hMSNs), establishing a hierarchically structured MIMBs with the sustained acoustic activity. Strong affinity between hMSNs and the gas-liquid interface facilitates cavitation effect transmission. Under low intensity ultrasound (<3 W/cm2) irradiation, primary MIMBs collapse generates secondary daughter bubbles that rapidly stabilize via hMSNs-mediated gas-liquid interface reconstruction and are able to cavitate again. This process enables energy-cascaded cavitation-successive bubble generations persisting until acoustic energy dissipation, achieving prolonged cavitation duration versus conventional lipid-shelled microbubbles. The sequential acoustomechanical perturbation generated by DOX-MIMBs induced synergistic tumor therapy: selective vascular destruction for mechanically collapsed immature tumor vasculature and enhanced chemotherapy for wider distribution and deeper penetration of DOX in tumors. Utilizing sequential bubble cavitation-induced shockwave and microstreaming, by integrating tumor vasculature mechanical disruption and deep tumor DOX penetration chemotherapy, DOX-MIMBs achieved tumor volume appropriate 90 % reduction in renal cell carcinoma models. Such elaborated DOX-MIMBs mechano-pharmaceutical delivery system achieve a paradigm shift from systemic drug bombardment to local mechanochemical tumor suppression and provide a powerful strategy for tumor precision therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


