纤维化胶原靶向递送系统阻断胰腺细胞间串扰减轻胰腺纤维化
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
胰腺纤维化(PF)是胰腺内稳态的显著破坏,主要特征是胰腺腺泡细胞(PAC)损伤、胰腺星状细胞(PSC)激活和持续炎症反应导致的细胞外基质(ECM)过度沉积。作为慢性胰腺炎和胰腺癌的标志性特征,进行性纤维化加剧了疾病的严重程度,并带来了重大的临床挑战。值得注意的是,活化的PSCs和受损的PACs之间的自我放大串扰使纤维形成永久化,破坏了治疗效果。然而,目前还没有有效的策略来调节细胞间质和减轻胰腺纤维化。在此,我们开发了双重载药脂质纳米颗粒(JM-CCs),其表面具有胶原结合肽(CBP)和胶原酶I。这种设计通过穿透致密的ECM屏障到达纤维化病灶的核心,促进了靶向药物的递送。释放的褪黑激素减轻PACs的氧化应激,从而减少其对psc的纤维化刺激。同时,包封的JTE013通过调节自噬抑制PSC的激活,导致ECM产生减少,减轻PAC损伤。在小蛋白诱导的PF小鼠模型中,jm - cc有效减少ECM沉积,修复胰腺外分泌功能。该研究为恢复胰腺组织稳态提供了一种新的策略,并为纤维化和胰腺疾病的治疗提供了一种有希望的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
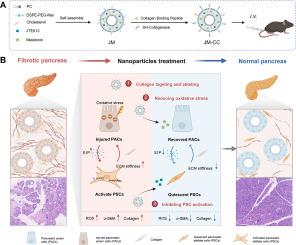
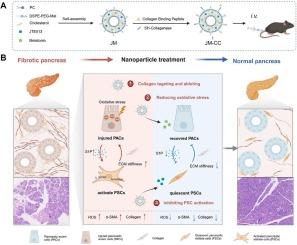
Fibrotic collagen-targeted delivery system blocks pancreatic intercellular crosstalk to alleviate pancreatic fibrosis
Pancreatic fibrosis (PF) is a significant disruption of homeostasis in the pancreas, primarily characterized by excessive extracellular matrix (ECM) deposition due to pancreatic acinar cell (PAC) injury, pancreatic stellate cell (PSC) activation, and persistent inflammatory response. As a hallmark feature of both chronic pancreatitis and pancreatic cancer, progressive fibrosis exacerbates disease severity and poses significant clinical challenges. Notably, the self-amplifying crosstalk between activated PSCs and injured PACs perpetuates fibrogenesis and undermines therapeutic efficacy. However, currently, no effective strategy is available to modulate intercellular and alleviate pancreatic fibrosis. Herein, we developed dual drug-loaded lipid nanoparticles (JM-CCs) functionalized with a collagen-binding peptide (CBP) and collagenase I on their surface. This design facilitates targeted drug delivery by enabling penetration through the dense ECM barrier to the core of fibrotic lesions. The released melatonin alleviates oxidative stress in PACs, thereby reducing their fibrogenic stimulation of PSCs. Concurrently, the encapsulated JTE013 suppresses PSC activation by modulating autophagy, leading to decreased ECM production and mitigation of PAC injury. In a caerulein-induced PF mouse model, JM-CCs effectively reduce ECM deposition and repair pancreatic exocrine function. This study provides a novel strategy for regaining pancreatic tissue homeostasis and offers a promising approach for therapies of fibrosis and pancreatic disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


