纳米粒子驱动的活性氧疗法:骨肉瘤治疗的新前沿
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
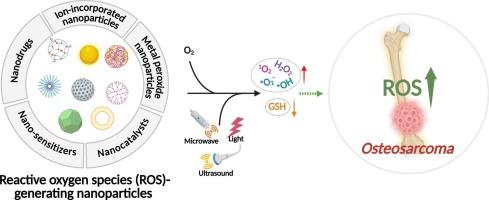
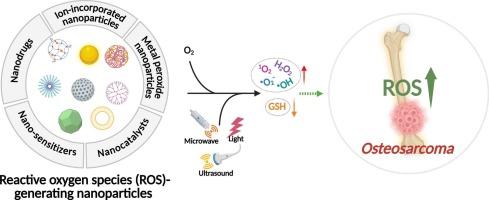
活性氧(ROS)在骨肉瘤(OS)治疗中发挥关键作用,当其水平超过细胞阈值时,可诱导程序性细胞死亡。由于其固有的ROS水平升高,骨肉瘤细胞比正常骨细胞更容易受到ROS上调的影响,这为选择性消除癌细胞提供了一个治疗窗口。然而,OS细胞固有的抗氧化防御机制减轻了常规ros生成剂的作用,限制了其临床疗效。为了克服这一挑战,研究人员开发了基于纳米颗粒的策略来增强活性氧的产生并改善治疗效果。尽管取得了重大进展,但设计和优化用于OS治疗的ros生成纳米平台的综合框架仍然缺乏。本文根据其潜在的作用机制对ros上调纳米颗粒进行了系统的分类,并讨论了它们的治疗潜力。此外,强调了关键挑战和未来方向,以指导下一代OS治疗纳米材料的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nanoparticle-driven reactive oxygen species therapy: A new frontier in osteosarcoma treatment
Reactive oxygen species (ROS) play a critical role in osteosarcoma (OS) therapy by inducing programmed cell death when their levels exceed the cellular threshold. Due to their inherently elevated ROS levels, OS cells are more susceptible to ROS upregulation than normal bone cells, offering a therapeutic window for selective cancer cell elimination. However, the intrinsic antioxidant defense mechanisms in OS cells mitigate the effects of conventional ROS-generating agents, limiting their clinical efficacy. To overcome this challenge, nanoparticle-based strategies have been developed to enhance ROS production and improve therapeutic outcomes. Despite significant progress, a comprehensive framework for designing and optimizing ROS-generating nanoplatforms for OS treatment remains lacking. This review systematically classifies ROS-upregulating nanoparticles based on their underlying mechanisms of action and discusses their therapeutic potential. Additionally, key challenges and future directions are highlighted to guide the development of next-generation nanomaterials for OS therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


