在氧化还原动态水环境下,增强文拉法辛在无定形FeS2中的降解:柠檬酸盐在促进羟基自由基生成的电子利用中的关键作用
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
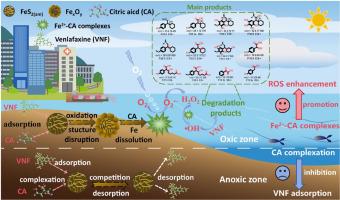
药物和有机酸在城市河流环境中普遍存在,但抗抑郁药如文拉法辛(VNF)在富含硫化铁的沉积物中氧化还原动力学条件下的转化机制尚不清楚。本研究全面分析了VNF在无定形硫化铁(FeS2(am))中的吸附/解吸动力学和活性氧(ROS)介导的氧化降解,强调了柠檬酸(CA)在调节这些相互作用中的作用。结果表明,VNF在缺氧条件下稳定吸附在FeS2(am)上,无明显降解。相反,暴露于O2会引发VNF的快速解吸(93.58%)和羟基自由基(•OH)介导的部分降解(16.96%)。值得注意的是,CA的添加显著促进了氧化条件下VNF的降解,降解率达到97.36%,观察到的速率常数(kobs)增加了17.4倍(从6.38×10−4到1.11×10−2 min−1)。机制上,CA通过三个协同途径优化了FeS2(am)的电子利用效率:(i)通过类芬顿链式反应产生以碳为中心的自由基,放大了secondary•OH的产生;(ii)通过增强硫中间体(如S2−,S0)到Fe3+的电子转移加速Fe2+再生;(3) H2O2转化为•OH的效率由57.20%提高到83.20%。电化学分析证实,CA实现了更有效的四电子O2还原途径,从而提高了快速生成•OH的电子利用率。密度泛函理论计算结合LC-Orbitrap-HRMS分析确定了9种不同的VNF降解途径。此外,ECOSAR结果表明,与母体化合物相比,转化产物的生态毒性显著降低。总的来说,这些发现为开发配体增强的抗抑郁污染物在氧化还原动力沉积物中的原位修复策略铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhanced venlafaxine degradation in amorphous FeS2 under redox-dynamic aqueous environments: Critical role of citrate in electron utilization for boosted hydroxyl radical generation
Pharmaceuticals and organic acids ubiquitously coexist in urban riverine environments, yet the transformation mechanisms of antidepressants like venlafaxine (VNF) in iron sulfide-rich sediments under redox-dynamic conditions remain poorly resolved. This study comprehensively analyzed adsorption/desorption dynamics and reactive oxygen species (ROS)-mediated oxidative degradation of VNF in amorphous iron sulfide (FeS2(am)), emphasizing the role of citric acid (CA) in modifying these interactions. Our results revealed that VNF was stably adsorbed onto FeS2(am) under anoxic conditions with no observable degradation. Conversely, exposure to O2 triggered rapid desorption of VNF (93.58%) and its partial degradation (16.96%) mediated by hydroxyl radicals (•OH). Remarkably, CA amendment significantly promoted VNF degradation under oxic conditions, achieving 97.36% degradation and increasing the observed rate constant (kobs) 17.4-fold (from 6.38×10−4 to 1.11×10−2 min−1). Mechanistically, CA optimized electron utilization efficiency in FeS2(am) through three synergistic pathways: (i) generation of carbon-centered radicals that amplified secondary •OH production via Fenton-like chain reactions; (ii) acceleration of Fe2+ regeneration through enhanced electron transfer from sulfur intermediates (e.g., S2−, S0) to Fe3+; and (iii) elevation of H2O2 into •OH conversion efficiency from 57.20% to 83.20%. Electrochemical analyses corroborated that CA enabled a more efficient four-electron O2 reduction pathway, thereby enhancing electron utilization for rapid •OH generation. Density functional theory calculations combined with LC-Orbitrap-HRMS analyses identified nine distinct VNF degradation pathways. Additionally, ECOSAR results indicated a significant reduction in ecotoxicity of the transformation products compared to the parent compound. Collectively, these findings pave the way for developing ligand-enhanced in situ remediation strategies for antidepressant contaminants in redox-dynamic sediments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


