衰老成纤维细胞分泌组对卵巢癌传播的影响。
IF 4.3
引用次数: 0
摘要
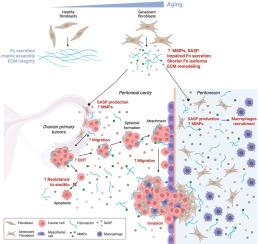
卵巢癌是最致命的妇科恶性肿瘤之一,衰老是卵巢癌的重要危险因素。超过50 %的卵巢癌病例发生在65岁及以上的女性中。在衰老过程中,衰老细胞在各种组织中逐渐积累,导致肿瘤微环境的结构和炎症变化。虽然最近的研究强调了衰老间皮细胞在促进癌细胞扩散中的作用,但结缔组织的主要组成部分衰老成纤维细胞的作用仍然难以捉摸。在这项研究中,我们首先表征了人类卵巢成纤维细胞的衰老表型,重点关注细胞外基质(ECM)重塑和衰老相关分泌表型(SASP)。衰老的卵巢成纤维细胞表现出细胞内纤维连接蛋白(Fn)的积累,纤维连接蛋白进入ECM组装受损,ECM组织明显改变。SASP分析揭示了一个富含免疫调节细胞因子和可能参与癌细胞传播和转移的因子的促炎分泌组。然后我们评估了衰老成纤维细胞分泌组对卵巢癌细胞行为的影响。虽然它不改变癌细胞的增殖,但它可以抵抗细胞凋亡,并显著增强癌细胞的迁移和侵袭。总之,我们的研究结果强调了衰老的卵巢成纤维细胞在促进癌细胞传播中的作用,并表明年龄相关的基质改变有助于疾病进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Impact of senescent fibroblasts secretome in ovarian cancer dissemination
Aging is a significant risk factor in ovarian cancers, which remains one of the most lethal gynecological malignancies. Over 50 % of ovarian cancer cases occur in women aged 65 and older. During aging, senescent cells progressively accumulate in various tissues, contributing to structural and inflammatory changes in the tumor microenvironment. While recent studies have underscored the role of the senescent mesothelial cells in facilitating cancer cell dissemination, the contribution of senescent fibroblasts that are a major component of the connective tissue, remains elusive. In this study, we first characterized the senescent phenotype of human ovarian fibroblasts, with a focus on extracellular matrix (ECM) remodeling and the senescence-associated secretory phenotype (SASP). Senescent ovarian fibroblasts exhibited an accumulation of intracellular Fibronectin (Fn), impaired fibrillar Fn into ECM assembly, and marked alterations in ECM organization. SASP profiling revealed a pro-inflammatory secretome enriched in immune-modulatory cytokines and factors potentially involved in cancer cell dissemination and metastasis. We then evaluated the influence of the senescent fibroblast secretome on ovarian cancer cell behavior. While it did not alter cancer cell proliferation, it conferred resistance to apoptosis and significantly enhanced cancer cell migration and invasion. Altogether, our findings highlight the role of senescent ovarian fibroblasts in promoting cancer cell dissemination and suggest that age-related stromal changes contribute to disease progression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental gerontology
Ageing, Biochemistry, Geriatrics and Gerontology
CiteScore
6.70
自引率
0.00%
发文量
0
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


