聚乙烯醇低温冰箱:聚合物浓度、循环次数和解冻速率对材料性能和皮肤给药的影响大小。
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-09-28
DOI:10.1016/j.ejpb.2025.114876
引用次数: 0
摘要
聚乙烯醇(PVA)可以通过反复的冻融(F-T)循环进行物理交联,从而形成冷冻凝胶。尽管冷冻过程在科学文献中越来越普遍,但其多变量性质,特别是工艺变量对材料特性的单个和协同影响,仍未得到充分探索。本研究首次系统分析了关键工艺参数聚合物浓度、循环次数和f - t循环内的解冻速率对PVA冷冻箱材料性能的影响。PVA冷冻箱是一种用于皮肤的给药系统。分析的变量与杨氏模量、凝胶强度和计算出的聚合物网络的网状尺寸有不同程度的正相关和负相关。此外,它独特地展示了单个工艺参数之间的协同组合效应,揭示了相互影响,这是以前没有系统表征的。以双氯芬酸钠为模型药物,通过比较药物释放和药物通过皮肤的渗透也证明了双氯芬酸钠作为皮肤给药系统的优异适用性。总的来说,这项研究首次系统地了解了冷冻工艺参数对材料性能的影响,并引入了一种新的简单方法来单独选择特定的冷冻条件。本文章由计算机程序翻译,如有差异,请以英文原文为准。
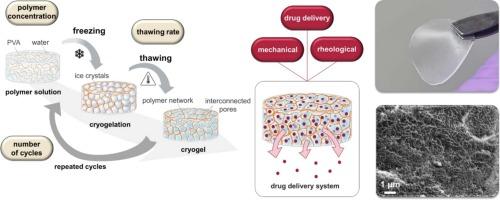
Poly(vinyl alcohol) cryogels: Effect size of polymer concentration, number of cycles and thawing rate on material properties and dermal drug delivery
Poly(vinyl alcohol) (PVA) can be physically cross-linked by repeated freeze–thaw (F-T) cycles, resulting in a cryogel. Despite the growing prevalence of the cryogelation process in the scientific literature, its multivariable nature, particularly the individual and synergistic impact of process variables on material properties, remains under-explored. The present work is the first systematic analysis quantifying the effect sizes associated with the influence of the key process parameters polymer concentration, number of cycles and thawing rates within F-T-cycles on the material properties of PVA cryogels as a drug delivery system for dermal applications. The variables analysed showed both positive and negative correlations with Young’s modulus, gel strength and calculated mesh sizes of the polymer network to varying degrees. Moreover, it uniquely demonstrates the synergistic combination effects between individual process parameters, revealing mutual influences, that had not been characterized systematically before. The excellent suitability as a dermal drug delivery system was also demonstrated by both comparative drug release and drug permeation through the skin using diclofenac sodium as a model drug. Overall, this study provides the first systematic understanding of the impact of cryogelation process parameters on material properties and introduces a new and simple approach to individual selection of specific cryogelation conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


