基于海胆样PdAg和Au NPs/N-C@CNTs的电化学免疫传感器用于CEA的超灵敏检测。
IF 4.5
2区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
癌胚抗原(CEA)是临床重要的肿瘤生物标志物,可用于早期癌症筛查和诊断。在这里,我们描述了一个三明治结构的电化学免疫传感平台,利用海胆样PdAg纳米结构和Au NPs/N-C@CNTs底物的协同信号放大,实现了超灵敏的CEA定量。PdAg的海胆状形态赋予了材料额外的过氧化氢还原催化活性位点,具有显著的电化学性能。此外,PdAg具有良好的生物相容性,可以有效地固定二抗。将多多巴胺包被的碳纳米管碳化生成氮掺杂碳纳米管(N-C@CNTs),碳纳米管通过稳定的AuN键与金纳米粒子(Au NPs)结合,从而促进了随后一抗与Au NPs的结合。优化后的分析方法具有较宽的动态范围(50 fg mL-1-100 ng mL-1),低检出限(1.04 fg mL-1, S/N = 3),以及出色的重现性、选择性和稳定性。该平台对早期肿瘤生物标志物的筛选具有重要的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
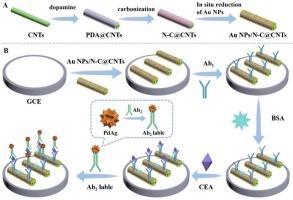
Electrochemical immunosensor based on sea urchin-like PdAg and Au NPs/N-C@CNTs for ultrasensitive detection of CEA
Carcinoembryonic antigen (CEA), a clinically critical tumor biomarker, enables early cancer screening and diagnosis. Here, we describe a sandwich-structured electrochemical immunosensing platform enabling supersensitive CEA quantification, leveraging synergistic signal amplification by sea urchin-like PdAg nanostructures and Au NPs/N-C@CNTs substrates. The urchin-like morphology of PdAg endows the material additional catalytic active sites for hydrogen peroxide reduction, which has remarkable electrochemical performance. Moreover, PdAg with superior biocompatibility can effectively immobilize the secondary antibody. Polydopamine-coated carbon nanotubes are carbonized to yield nitrogen-doped carbon nanotubes (N-C@CNTs), which are bound to gold nanoparticles (Au NPs) via stable Au![]() N bonds, thereby facilitating the subsequent binding of primary antibodies to the Au NPs. Optimized assays demonstrated a broad dynamic range (50 fg mL−1–100 ng mL−1) with low detection limits (1.04 fg mL−1, S/N = 3), coupled with exceptional reproducibility, selectivity, and stability. This platform holds significant promise for the screening of early-stage tumor biomarkers.
N bonds, thereby facilitating the subsequent binding of primary antibodies to the Au NPs. Optimized assays demonstrated a broad dynamic range (50 fg mL−1–100 ng mL−1) with low detection limits (1.04 fg mL−1, S/N = 3), coupled with exceptional reproducibility, selectivity, and stability. This platform holds significant promise for the screening of early-stage tumor biomarkers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioelectrochemistry
生物-电化学
CiteScore
9.10
自引率
6.00%
发文量
238
审稿时长
38 days
期刊介绍:
An International Journal Devoted to Electrochemical Aspects of Biology and Biological Aspects of Electrochemistry
Bioelectrochemistry is an international journal devoted to electrochemical principles in biology and biological aspects of electrochemistry. It publishes experimental and theoretical papers dealing with the electrochemical aspects of:
• Electrified interfaces (electric double layers, adsorption, electron transfer, protein electrochemistry, basic principles of biosensors, biosensor interfaces and bio-nanosensor design and construction.
• Electric and magnetic field effects (field-dependent processes, field interactions with molecules, intramolecular field effects, sensory systems for electric and magnetic fields, molecular and cellular mechanisms)
• Bioenergetics and signal transduction (energy conversion, photosynthetic and visual membranes)
• Biomembranes and model membranes (thermodynamics and mechanics, membrane transport, electroporation, fusion and insertion)
• Electrochemical applications in medicine and biotechnology (drug delivery and gene transfer to cells and tissues, iontophoresis, skin electroporation, injury and repair).
• Organization and use of arrays in-vitro and in-vivo, including as part of feedback control.
• Electrochemical interrogation of biofilms as generated by microorganisms and tissue reaction associated with medical implants.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


