具有酰胺信号放大的化学发光光纤生物传感平台用于肿瘤源性外泌体的超灵敏检测。
IF 10.5
1区 生物学
Q1 BIOPHYSICS
引用次数: 0
摘要
外泌体已成为液体活检中有前途的非侵入性生物标志物,用于癌症的早期诊断和治疗。传统的外泌体分析方法往往受到复杂的程序和昂贵的仪器的限制,以及包括低信号分化和体液中大量游离蛋白的干扰等挑战。在此,我们提出了一种新的生物传感平台,该平台将酪酰胺信号放大(TSA)与脂质锚定的化学发光光纤传感器(COFS)集成在一起。该平台利用二硬脂酰磷脂酰乙醇胺-聚乙二醇(DSPE-PEG)作为脂质锚来捕获外泌体和辣根过氧化物酶(HRP)标记的适配体进行特异性识别。表面蛋白和膜结构的双重识别避免了游离蛋白的干扰。基于tsa的信号放大系统显著提高了检测灵敏度,这得益于外泌体膜中丰富的蛋白质作为活性酪胺的沉积位点。提出的TSA-COFS平台为外泌体定量的挑战提供了一种经济有效的解决方案,实现了生物样品中肿瘤来源外泌体的快速分析、超灵敏检测和即时分析。通过分析人类临床血清样本中表达Mucin 1蛋白(MUC1)的外泌体,证实了该平台的有效性。结果表明,该方法能够快速准确地测定外泌体,具有较高的选择性和极低的检出限(6.76粒/μL)。一个自制的便携式设备,配备传感器探针,“插入式”操作试剂条,电池供电的光子计数器和触摸屏计算机,用于分析,展示了TSA-COFS在外泌体定量和点护理测试(POCT)应用中的潜在实用价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
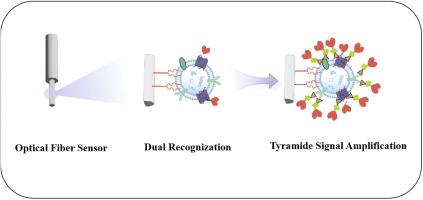
Chemiluminescent optical fiber biosensing platform with tyramide signal amplification for ultrasensitive detection of tumor-derived exosomes
Exosomes have emerged as promising non-invasive biomarkers in liquid biopsy for the early diagnosis and treatment of cancers. Traditional methods for exosome analysis are often limited by complex procedures and costly instruments, as well as challenges including low signal differentiation and interference from large amounts of free proteins in body fluids. Herein, we propose a novel biosensing platform that integrates tyramide signal amplification (TSA) with a lipid-anchored chemiluminescent optical fiber sensor (COFS). The platform utilizes distearoyl phosphatylethanolamine-polyethylene glycol (DSPE-PEG) as lipid-anchor to capture exosomes and horseradish peroxidase (HRP)-labeled aptamers for specific recognition. The dual identification of surface proteins and membrane structure avoids interference from free proteins. The TSA-based signal amplification system significantly enhances detection sensitivity, benefiting from the abundance of proteins in exosomal membranes that function as deposition sites for active tyramine. The proposed TSA-COFS platform offers a cost-effective solution to the challenges of exosome quantitation, enabling rapid analysis, ultrasensitive detection and point-of-care profiling of tumor-derived exosomes within biological samples. The efficacy of the platform was demonstrated by analyzing Mucin 1 protein (MUC1)-expressing exosomes from human clinical serum samples. The results show that the method can achieve rapid and accurate determination of exosomes with high selectivity and an extremely low limit-of-detection of 6.76 particles/μL. A home-made portable device, equipped with the sensor probe, a "plug-in" operation of the reagent strip, a battery-powered photon counter and a touch-screen computer, is used for the assays, demonstrating the potential practical value of the TSA-COFS for exosome quantitation and point-of-care testing (POCT) applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biosensors and Bioelectronics
工程技术-电化学
CiteScore
20.80
自引率
7.10%
发文量
1006
审稿时长
29 days
期刊介绍:
Biosensors & Bioelectronics, along with its open access companion journal Biosensors & Bioelectronics: X, is the leading international publication in the field of biosensors and bioelectronics. It covers research, design, development, and application of biosensors, which are analytical devices incorporating biological materials with physicochemical transducers. These devices, including sensors, DNA chips, electronic noses, and lab-on-a-chip, produce digital signals proportional to specific analytes. Examples include immunosensors and enzyme-based biosensors, applied in various fields such as medicine, environmental monitoring, and food industry. The journal also focuses on molecular and supramolecular structures for enhancing device performance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


