靶向半胱氨酸828的FLT3共价抑制剂8-苯基喹唑啉-2胺衍生物的设计、合成及生物学评价
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
靶向fms样酪氨酸激酶3 (FLT3)的致癌激活突变已成为治疗急性髓性白血病(AML)的一种有前景的治疗方法。然而,快速发展的耐药性已经显著降低了FLT3抑制剂的临床疗效和治疗耐久性。共价抑制剂在克服耐药性方面显示出令人印象深刻的潜力。在此,我们设计并合成了一系列8-苯基喹唑啉-2-胺衍生物作为FLT3共价抑制剂靶向半胱氨酸828。其中,4k对FLT3-ITD阳性AML细胞和含有耐药FLT3-ITD继发性突变的BaF3细胞(包括BaF3-FLT3-ITD- d835v /I)表现出有效的选择性抑制活性。生化和质谱分析证实,4k共价结合在FLT3的ATP口袋中的Cys828。4k还能抑制FLT3及其下游信号因子的磷酸化,诱导细胞周期阻滞和凋亡。此外,4k的口服生物利用度为12.48%,可有效抑制MV4-11异种移植瘤模型的肿瘤生长,且无明显毒性。综上所述,4k代表了一种靶向FLT3激酶Cys828的新型共价抑制剂,可用于AML的靶向治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
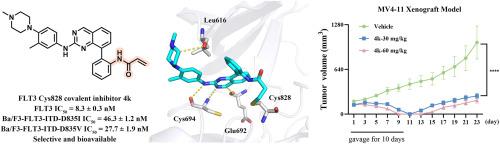
Design, synthesis and biological evaluation of 8-phenylquinazolin-2-amine derivatives as FLT3 covalent inhibitors targeting cysteine 828 in the ATP pocket
Targeting oncogenic activating mutations of Fms-Like tyrosine kinase 3 (FLT3) has constituted a promising therapy for acute myeloid leukemia (AML). However, rapid development of resistance has significantly compromised clinical efficacy and therapeutic durability of FLT3 inhibitors. Covalent inhibitors have shown impressive potential in overcoming drug resistance. Herein, we designed and synthesized a series of 8-phenylquinazolin-2-amine derivatives as FLT3 covalent inhibitors targeting cysteine 828. Among them, 4k demonstrated potent and selective inhibitory activities against FLT3-ITD positive AML cells and BaF3 cells harboring drug-resistant FLT3-ITD secondary mutations, including BaF3-FLT3-ITD-D835V/I. Biochemical and mass spectrometry analyses confirmed that 4k covalently bound to the Cys828 in the ATP pocket of FLT3. 4k also inhibited the phosphorylation of FLT3 and its downstream signaling factors, as well as induced cell cycle arrest and apoptosis. Furthermore, 4k, with an oral bioavailability of 12.48%, effectively suppressed tumor growth in a MV4-11 xenograft model without obvious toxicity. Taken together, 4k represents a novel covalent inhibitor targeting Cys828 of FLT3 kinase for targeted therapy of AML.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


