线粒体靶向I/II型自我报告光敏剂,具有黏度敏感特性,用于肿瘤识别和细胞凋亡诱导
IF 3.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
建立光动力治疗(PDT)过程与线粒体黏度变化之间的动态相关性,对于推进肿瘤治疗技术的发展具有重要的研究价值。然而,在单分子尺度上构建线粒体粘度敏感探针,同时对肿瘤具有高I/II型PDT疗效和可视化治疗效果的研究仍然缺乏。本研究以四苯基乙烯(TPE)取代咔唑为供电子基团(D),碘化吲哚盐为供电子组分(a),制备了一种新型D-π-A型有机小分子光敏剂(TKC)。TKC能够在宽粘度范围内(η, 1.005-1410 cP)进行高灵敏度和特异性荧光成像,实现细胞内线粒体粘度的实时原位成像以及正常细胞和癌细胞之间的准确区分。此外,TKC具有高效的I/II型ROS生成能力,其1O2量子产率为3.56,同时在荷瘤小鼠中对肿瘤细胞具有有效的光毒性并抑制肿瘤生长。更重要的是,TKC可以监测线粒体去极化过程和细胞凋亡过程中线粒体粘度的动态变化,实现对治疗效果的实时跟踪和自我报告。因此,本研究开发的集肿瘤精确诊断、线粒体靶向治疗和动态疗效评价为一体的荧光探针,为后续发展综合诊断和治疗荧光工具提供了有效的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
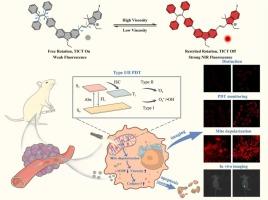
Mitochondrial-targeted type I/II self-reporting photosensitizer with viscosity-sensitive properties for tumor recognition and apoptosis induction
Establishing a dynamic correlation between photodynamic therapy (PDT) processes and changes in mitochondrial viscosity is of significant research value for advancing the development of theranostics technologies for tumors. Nevertheless, research on constructing mitochondrial viscosity-sensitive probes at the single-molecule scale that simultaneously possess high type I/II PDT efficacy for tumors and the ability to visualize therapeutic effects remains lacking. In this work, we developed a novel D-π-A type organic small molecule photosensitizer (TKC), constructed by substituting carbazole with tetraphenylethylene (TPE) as the electron-donating moiety (D) and employing indole iodide salt as the electron-accepting component (A). TKC is capable of performing highly sensitive and specific fluorescence imaging over a wide viscosity range (η, 1.005–1410 cP), enabling real-time in situ imaging of intracellular mitochondrial viscosity as well as accurate differentiation between normal and cancer cells. Furthermore, TKC exhibits efficient type I/II ROS generation capabilities, with a 1O2 quantum yield of 3.56, while achieving efficient phototoxicity to tumor cells and inhibition of tumor growth in tumor-bearing mice. More importantly, TKC can monitor the mitochondrial depolarization process and the dynamic changes of mitochondrial viscosity during the process of apoptosis, enabling real-time tracking and self-reporting of treatment effects. Therefore, the fluorescent probe developed in this work, which integrates precise tumor diagnosis, mitochondrial-targeted therapy and dynamic efficacy evaluation, offers an effective strategy for the subsequent advancement of integrated diagnostic and therapeutic fluorescent tools.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sensors and Actuators B: Chemical
工程技术-电化学
CiteScore
14.60
自引率
11.90%
发文量
1776
审稿时长
3.2 months
期刊介绍:
Sensors & Actuators, B: Chemical is an international journal focused on the research and development of chemical transducers. It covers chemical sensors and biosensors, chemical actuators, and analytical microsystems. The journal is interdisciplinary, aiming to publish original works showcasing substantial advancements beyond the current state of the art in these fields, with practical applicability to solving meaningful analytical problems. Review articles are accepted by invitation from an Editor of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


