幽门螺杆菌衍生的外膜囊泡:致病作用、微生物群相互作用和生物医学应用
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
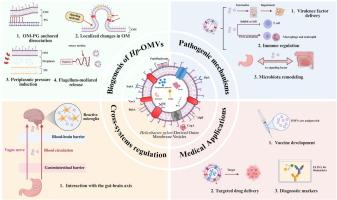
幽门螺杆菌(Hp)是一种革兰氏阴性的微嗜气细菌,感染了全球约45%的人口。它是慢性胃炎、消化性溃疡和胃癌的主要诱因。在其分泌产物中,hp衍生的外膜囊泡(Hp-OMVs)是纳米级蛋白脂质体结构,在宿主-病原体相互作用中起着至关重要的作用。本文综述了hp - omv的形成、组成和生物学功能,重点介绍了它们的系统作用和潜在的生物医学应用。whp - omv富含毒力相关蛋白和脂质,作为促进毒素递送、调节宿主免疫以及重塑胃肠道和口腔微生物群落的多功能载体。新出现的临床前研究表明,hp - omv可能会越过上皮屏障转移到远处的器官,包括大脑,在阿尔茨海默病的小鼠模型中,hp - omv已被证明会加剧神经炎症反应。然而,在人类中没有发现因果关系。迫切需要更多的研究来证实这种肠胃外的联系。同时,由于其固有的免疫原性和组织亲和性,hp - omv正被探索作为疫苗开发、粘膜佐剂、抗粘连治疗和靶向药物递送系统的通用平台。结论Hp- omv是Hp发病机制中一种独特的囊泡形态,具有广阔的生物医学潜力。未来的研究需要采用多组学、单囊泡分析和临床验证来确定它们的机制作用和转化价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Helicobacter pylori-derived outer membrane vesicles: Pathogenic roles, microbiota interactions, and biomedical applications
Background
Helicobacter pylori (Hp) is a gram-negative, microaerophilic bacterium that infects approximate 45% of the global population. It is a key contributor to chronic gastritis, peptic ulcers, and gastric cancer. Among its secreted products, Hp-derived outer membrane vesicles (Hp-OMVs) are nanoscale proteoliposomal structures that play crucial roles in host-pathogen interactions.Aim of Review
This review aims to synthesize current knowledge on the formation, composition, and biological functions of Hp-OMVs, with a particular focus on their systemic effects and potential biomedical applications.Key Scientific Concepts of Review
Hp-OMVs are enriched with virulence-associated proteins and lipids, acting as multifunctional vehicles that facilitate the delivery of toxins, modulation of host immunity, and reshaping of gastrointestinal and oral microbial communities.Emerging preclinical studies suggest that Hp-OMVs may translocate across epithelial barriers and reach distant organs, including the brain, where they have been shown to exacerbate neuroinflammatory responses in murine models of Alzheimer’s disease. However, no causal relationship has been demonstrated in humans. More research is urgently needed to confirm such extragastrointestinal connections.In parallel, Hp-OMVs are being explored as versatile platforms for vaccine development, mucosal adjuvants, anti-adhesion therapies, and targeted drug delivery systems, owing to their intrinsic immunogenicity and tissue tropism.Conclusion
Hp-OMVs represent a unique vesicular modality in Hp pathogenesis with broad biomedical potential. Future research employing multi-omics, single-vesicle analytics, and clinical validation is needed to define their mechanistic roles and translational value.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


