黑色素瘤mhc - i膜包裹Cu@ferrihydrite诱导铁下垂/铜下垂和对肿瘤的系统性免疫
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
主要组织相容性复合体I (MHC-I)在增强癌症免疫原性和免疫识别方面具有重要的潜力。在这里,我们报告了一种创新的治疗策略,将蓝光上调的MHC-I表达与蓝光诱导的铁下垂和铜下垂协同整合。蓝光通过调节NF-κB-SUSD6信号轴促进小鼠黑色素瘤细胞MHC-I的表达。随后,一个mhc - i富集的黑色素瘤细胞膜被用来包封光响应Cu@ferrihydrite (Cu@Fh)纳米粒子,形成M-Cu@Fh。mhc - 1促进了树突状细胞(dc)的成熟和CD8+/CD4+ T细胞的活化。M-Cu@Fh也通过蓝光照射下Fe/Cu离子的可控释放引发氧化应激和并发铁下垂/铜下垂。体内实验表明,蓝光和M-Cu@Fh的结合将免疫“冷”肿瘤转化为“热”肿瘤,通过氧化损伤抑制原位黑色素瘤生长,增强免疫原性。此外,在淋巴器官(淋巴结和脾脏)和肺中,DCs和CD8+/CD4+ T细胞的全身激活赋予了预防体外转移的作用。我们的研究阐明了黑色素瘤细胞中MHC-I的光调节机制,并提出了一种变变性的组合策略,即蓝光驱动光免疫疗法(PIT)与蓝光激活光动力疗法(PDT)协同作用,用于黑色素瘤的治疗和转移预防。本文章由计算机程序翻译,如有差异,请以英文原文为准。
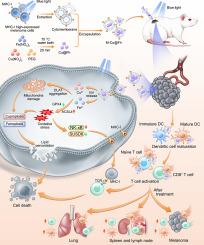
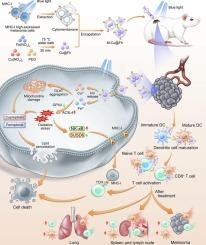
Melanoma MHC-I-membrane-encapsulated Cu@ferrihydrite induces ferroptosis/cuproptosis and systematic immunity against tumor
Major histocompatibility complex I (MHC-I) has significant potential for augmenting cancer immunogenicity and immune recognition. Here, we report an innovative therapeutic strategy that synergistically integrates blue light-upregulated MHC-I expression with blue light-induced ferroptosis and cuproptosis. Blue light promoted MHC-I expression in mouse melanoma cells by modulating the NF-κB-SUSD6 signaling axis. Subsequently, an MHC-I-enriched melanoma cytomembrane was used to encapsulate the photoresponsive Cu@ferrihydrite (Cu@Fh) nanoparticles, forming M-Cu@Fh. MHC-I facilitated dendritic cells (DCs) maturation and CD8+/CD4+ T cells activation. M-Cu@Fh also triggered oxidative stress and concurrent ferroptosis/cuproptosis through the controllable release of Fe/Cu ions under blue-light irradiation. In vivo experiments demonstrated that the combination of blue light and M-Cu@Fh converted immune “cold” tumors into “hot” tumors, suppressed in situ melanoma growth through oxidative damages and enhanced immunogenicity. Furthermore, systemic activation of DCs and CD8+/CD4+ T cells in lymphoid organs (lymph nodes and spleen) and lungs conferred prophylactic efficacy against abscopal metastasis. Our study elucidates the photoregulatory mechanism of MHC-I in melanoma cells and presents a transformative combinatorial strategy that synergizes blue light-driven photoimmunotherapy (PIT) with blue light-activated photodynamic therapy (PDT) for melanoma management and metastasis prevention.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


