微波辅助水热合成α-Mn2O3
IF 1.5
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
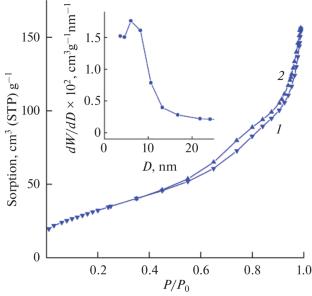
以高锰酸钾和抗坏血酸为原料,摩尔比为1∶(1 - 1.5),在空气中退火,采用微波辅助水热法合成立方α-Mn2O3。提出了氧化锰(III)形成的可能机理。采用粉末x射线衍射、扫描电镜和低温氮气吸附等方法对合成的α-Mn2O3进行了主要理化性质的测定。研究发现,通过改变反应混合物组分的摩尔比和前驱体的退火条件,可以得到β-MnO2、Mn3O4、MnO/C和Mn3O4/C复合材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Microwave-Assisted Hydrothermal Synthesis of α-Mn2O3
Cubic α-Mn2O3 was synthesized by microwave-assisted hydrothermal treatment of a reaction mixture containing potassium permanganate and ascorbic acid taken in a molar ratio of 1: (1–1.5) followed by annealing in air. A possible mechanism for the formation of manganese(III) oxide was proposed. The main physicochemical characteristics of the synthesized α-Mn2O3 were determined using powder X-ray diffraction, scanning electron microscopy, and low-temperature nitrogen adsorption. It was found that β-MnO2, Mn3O4, and MnO/C and Mn3O4/C composites can be additionally obtained by varying the molar ratio of the components of the reaction mixture and the conditions of annealing of the precursors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Russian Journal of Inorganic Chemistry
化学-无机化学与核化学
CiteScore
3.10
自引率
38.10%
发文量
237
审稿时长
3 months
期刊介绍:
Russian Journal of Inorganic Chemistry is a monthly periodical that covers the following topics of research: the synthesis and properties of inorganic compounds, coordination compounds, physicochemical analysis of inorganic systems, theoretical inorganic chemistry, physical methods of investigation, chemistry of solutions, inorganic materials, and nanomaterials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


