电渗析同时从LiCl中回收HCl和LiOH:建模、优化和实验验证
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
对锂离子电池(LIBs)日益增长的需求凸显了对可持续回收技术的需求。电渗析(EED)已成为一种很有前途的化学试剂再生和从湿法冶金渗滤液中回收锂基盐的替代方法。本研究研究了使用四室EED电池从LiCl溶液中电解生产HCl和LiOH。结合电化学和质量传递参数,建立了描述离子传输、酸碱再生和能量消耗的数学模型。通过5个不同操作条件下的批量EED实验对模型进行了验证。实验结果验证了该模型在预测Li+、Cl−、H+和OH−浓度随时间变化以及电压和比能量消耗方面的准确性,R2值均在0.99以上。在高初始LiOH浓度(0.5 M)和低电极距离(8 cm)的条件下,在保持高产品收率的同时最大限度地减少了能量消耗,达到了最佳性能。相反,较高的初始酸浓度降低离子回收,可能是由于离子竞争和膜运输阻力增加。在最优条件下,比能耗达到8.3 kWh·kg−1 HCl和13.7 kWh·kg−1 LiOH。经过验证的模型为优化EED性能和减少实验工作量提供了可靠的工具。这项工作证明了使用EED选择性地从富含锂离子的溶液中生成酸和碱的可行性,并支持开发更有效的锂离子电池回收方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
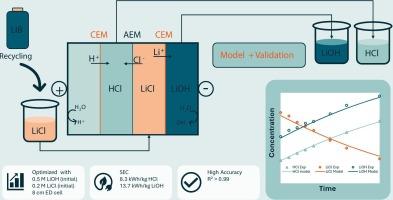
Simultaneous electrodialytic recovery of HCl and LiOH from LiCl: Modeling, optimization and experimental validation
The growing demand for lithium-ion batteries (LIBs) highlights the need for sustainable recycling technologies. Electro-electrodialysis (EED) has emerged as a promising alternative for regenerating chemical reagents and recovering lithium-based salts from hydrometallurgical leachates. This study investigates the electrodialytic production of HCl and LiOH from LiCl solutions using a four compartment EED cell. A mathematical model was developed to describe ions transports, acid and base regeneration and energy consumption, incorporating electrochemical and mass transport parameters. The model was validated through five batch-mode EED experiments under different operating conditions. Experimental results validated the model's accuracy in predicting the concentrations of Li+, Cl−, H+ and OH− over time, as well as the voltage and specific energy consumption, with R2 values above 0.99. The optimal performance was achieved under conditions of high initial LiOH concentration (0.5 M) and low electrode distance (8 cm), which minimized the energy consumption while maintaining high product yields. Conversely, higher initial acid concentrations reduced ion recovery, likely due to increased ionic competition and membrane transport resistance. Under the optimal conditions, specific energy consumption reached value8.3 kWh·kg−1 HCl and 13.7 kWh·kg−1 LiOH. The validated model provides a reliable tool for optimizing EED performance and reducing experimental effort. This work demonstrates the feasibility of using EED to selectively generate acid and base from LiCl-rich solutions and supports the development of more efficient approaches for LIB recycling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


