利用动力学分析和人工神经网络预测美洲龙舌兰生物废弃物纤维热降解
IF 6.2
1区 农林科学
Q1 AGRICULTURAL ENGINEERING
引用次数: 0
摘要
传统的动力学模型通常用于分析生物质纤维的热解行为。然而,它们的精度往往受到限制,因为它们难以捕捉热因素之间复杂的非线性相互作用。采用热重分析法和衍生热重分析法研究了不同升温速率(β = 5、10、15、20、25和30 °C/min)下美洲龙舌兰(Agave americana)废弃物花茎纤维的热解特性和热降解行为。结果表明,随着β的增加,两个脱挥发区明显向更高的温度移动,第一个峰从320°C(5 °C/min)移动到350°C(30 °C/min),第二个峰在相同的范围内从410°C移动到445°C。这种转变是由于在较高的β下传热效率降低,从而影响热降解动力学。建立了人工神经网络(ANN)模型,特别是结构(5 × 17 × 1)来模拟热解过程。人工神经网络模型的平均偏置误差小于2 %,平均绝对误差小于0.03,相关系数大于0.98,与实验数据吻合较好。与flynn - wallozawa、Kissinger-Akahira-Sunose和Starink方法得到的动力学参数比较表明,人工神经网络稍微高估了活化能,平均预测值为135 kJ/mol,而动力学方法的平均预测值为125-130 kJ/mol。人工神经网络还能有效地预测热力学参数,焓变值在120-140 kJ/mol之间,吉布斯自由能在110 kJ/mol左右,熵变值变化不大,与实验结果偏差较小。本文章由计算机程序翻译,如有差异,请以英文原文为准。
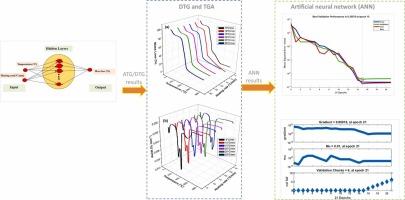
Predicting the thermal degradation of Agave americana L. biowaste fibers using kinetic analysis and artificial neural networks
Traditional kinetic models are commonly employed to analyze the pyrolysis behavior of biomass fibers. However, their accuracy is often limited because they struggle to capture the complex, nonlinear interactions among thermal factors. This study uses thermogravimetric analysis and derivative thermogravimetric analysis to examine the pyrolysis properties and thermal degradation behavior of flower stalk fibers from Agave americana waste under different heating rates (β = 5, 10, 15, 20, 25, and 30 °C/min). The results show a clear shift of both devolatilization zones to higher temperatures as β increases, with the first peak moving from 320°C at 5 °C/min to 350°C at 30 °C/min, and the second peak shifting from 410°C to 445°C over the same range. This shift is attributed to a decrease in heat transfer efficiency at higher β, which affects the thermal degradation kinetics. Artificial neural network (ANN) models, especially the architecture (5 × 17 × 1), were developed to model the pyrolysis process. The ANN model achieved a mean bias error of less than 2 %, a mean absolute error of less than 0.03, and a correlation coefficient of over 0.98, demonstrating strong agreement with experimental data. Comparisons with kinetic parameters obtained from Flynn-Wall-Ozawa, Kissinger-Akahira-Sunose, and Starink methods indicated that the ANN slightly overestimated activation energies, with average predicted values of 135 kJ/mol compared to 125–130 kJ/mol from kinetic methods. The ANN also effectively predicted thermodynamic parameters, with enthalpy change values between 120–140 kJ/mol, Gibbs free energy around 110 kJ/mol, and entropy change values that varied slightly with minor deviations from experimental results.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial Crops and Products
农林科学-农业工程
CiteScore
9.50
自引率
8.50%
发文量
1518
审稿时长
43 days
期刊介绍:
Industrial Crops and Products is an International Journal publishing academic and industrial research on industrial (defined as non-food/non-feed) crops and products. Papers concern both crop-oriented and bio-based materials from crops-oriented research, and should be of interest to an international audience, hypothesis driven, and where comparisons are made statistics performed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


