表皮水动力学控制着植物芽分生组织的水分稳态,以适应陆地环境
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
水分的吸收和再分配是植物在陆地上定植的一个重大挑战。虽然维管植物已经进化出专门的水运输结构,但如何在分生组织中维持水的稳态仍然是一个谜。在这里,我们表明拟南芥茎分生组织在高湿生态位中发育。同源结构域亮氨酸拉链(HD-ZIP)转录因子拟南芥分生系统层1 (ATML1)及其调控靶点PIP25 .在L1细胞间建立水管,促进与周围微环境的水力交换。ATML1-PIP2;5模块调节干细胞活动以应对湿度波动,并与自然种群对干旱气候的局部适应有关。IV类同源结构域亮氨酸拉链(C4HDZ)蛋白对水通量的转录激活早于血管系统的出现,有助于多形地草的水力反应。我们的研究结果揭示了一个进化上保守的表皮水力通路,它将发育模式与环境感知结合在一起,突出了茎分生组织在塑造植物适应陆地栖息地的基本作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
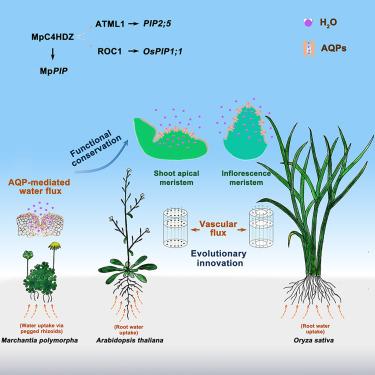
Epidermal hydrodynamics controls water homeostasis of shoot meristems for plant adaptation to terrestrial environments
Water uptake and redistribution represent a significant challenge for plant colonization of land. While vascular plants have evolved specialized structures for water transport, how water homeostasis is maintained in meristematic tissues remains elusive. Here, we show that the Arabidopsis shoot meristem develops within a high-humidity niche. The homeodomain leucine zipper (HD-ZIP) transcription factor ARABIDOPSIS THALIANA MERISTEM LAYER 1 (ATML1) and its regulatory target PIP2;5 establish a water conduit across L1 cells to facilitate hydraulic exchange with the surrounding microenvironment. The ATML1-PIP2;5 module regulates stem cell activity in response to humidity fluctuations and is associated with local adaptation to arid climates in natural populations. Transcriptional activation of water flux by class IV homeodomain-leucine zipper (C4HDZ) proteins predates the emergence of vascular systems, contributing to hydraulic response in the liverwort Marchantia polymorpha. Our results reveal an evolutionarily conserved epidermal hydraulic pathway that integrates developmental patterning with environmental sensing, highlighting a fundamental role for the shoot meristem in shaping plant adaptation in terrestrial habitats.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


