硫酸盐自由基驱动氧化与动态原位Fe(III)混凝的协同作用增强了对NOM前驱体的去除
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
通过常规混凝法去除天然有机物(NOM)往往是有限的。本研究研究了铁(II)激活的硫酸盐基氧化(Fe(II)@SBOs),包括过氧单硫酸盐和过氧二硫酸铁(Fe(II)@PMS和Fe(II)@PS),以增强对无机氮衍生消毒副产物(DBPs)的去除。我们为探索Fe(II)@SBOs中原位自由基氧化和快速凝固的协同作用提供了新的证据。在pH 7和低摩尔剂量的DOC:氧化剂:Fe为1:0.5:0.5时,Fe(II)@PMS和Fe(II)@PS对DOC(55-59 %)、含氟物质(64-71 %)和DBP前体(59-71 %)的去除效果更好,在所有测试参数中都比传统的Fe(III)高出约20-30 %。在1:2:2的高剂量下,两种Fe(II)@SBO系统都能减少80-90 % DBP前体,这使处理后的水的计算细胞毒性效力比未经处理的水低0.5至1个数量级。自由基鉴定证实,Fe(II)@PMS比Fe(II)@PS更有效地生成•OH和SO4•-自由基。这些自由基氧化了NOM,将其碳碳结构从低(如CC/CH, CO)转变为高氧化碳基(如CO, OCO)。它们通过前体的结构改变而不是完全的有机减缓来减少DBP的形成,这一点在清除实验中的轻微影响中得到了证明。相反,螯合实验表明,新形成的铁(III)以单体和聚合铁为特征,控制了NOM的去除过程。原位Fe(III)促进了快速混凝,使氧化后的NOM能更好地中和电荷,最终生成更致密的OO结构絮凝体。这些特性与传统的Fe(III)形成松散和细长的絮凝体相反。本研究阐述了Fe(II)@SBOs系统中原位氧化和混凝的协同作用,为缓解nomo - dbp提供了有希望的替代方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
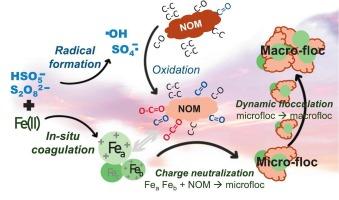
Synergy of sulfate radical-driven oxidation with dynamic in situ Fe(III) coagulation for enhanced NOM precursor removal
Removal of natural organic matter (NOM) by conventional coagulation is often limited. This study investigated Fe(II)-activated sulfate-based oxidations (Fe(II)@SBOs), including peroxymonosulfate and peroxydisulfate (Fe(II)@PMS and Fe(II)@PS), for enhanced removal of NOM-derived disinfection by-products (DBPs). We provided fresh evidence exploring the synergistic roles of in situ radical oxidation and swift coagulation in Fe(II)@SBOs. At pH 7 and a low molar dose of DOC:Oxidant: Fe of 1:0.5:0.5, Fe(II)@PMS and Fe(II)@PS achieved better removals in DOC (55–59 %), fluorophoric substances (64–71 %), and DBP precursors (59–71 %), outperforming traditional Fe(III) by approximately 20–30 % for all tested parameters. At a high dose of 1:2:2, both Fe(II)@SBO systems reduced 80–90 % DBP precursors, which mitigated the calculated cytotoxicity potency of treated water by 0.5 to 1 order of magnitude lower than untreated water. Radical identification confirmed that Fe(II)@PMS generated •OH and SO4•– radicals more effectively than Fe(II)@PS. These radicals oxidized NOM, transforming its carbon‑carbon structure from a low (i.e. C C/C
C/C H, C
H, C O) to a high degree of oxygenated carbonaceous groups (i.e. C
O) to a high degree of oxygenated carbonaceous groups (i.e. C O, O
O, O C
C O). They reduced DBP formation via structural alternation of the precursor rather than complete organic mitigation, as evidenced by their minor impact in scavenging experiments. Instead, the newly formed Fe(III) characterized by monomeric and polymeric iron, governed the NOM removal process as demonstrated by chelating experiments. In situ Fe(III) facilitated swift coagulation for better charge neutralization with oxidized NOM and ultimately produced more compact flocs with a O
O). They reduced DBP formation via structural alternation of the precursor rather than complete organic mitigation, as evidenced by their minor impact in scavenging experiments. Instead, the newly formed Fe(III) characterized by monomeric and polymeric iron, governed the NOM removal process as demonstrated by chelating experiments. In situ Fe(III) facilitated swift coagulation for better charge neutralization with oxidized NOM and ultimately produced more compact flocs with a O O structure. These characteristics were opposed to conventional Fe(III), which formed loose and elongated flocs. This study elaborates the synergistic roles of in situ oxidation and coagulation in Fe(II)@SBOs systems, offering promising alternatives to conventional coagulation for NOM-DBP mitigation.
O structure. These characteristics were opposed to conventional Fe(III), which formed loose and elongated flocs. This study elaborates the synergistic roles of in situ oxidation and coagulation in Fe(II)@SBOs systems, offering promising alternatives to conventional coagulation for NOM-DBP mitigation.
 C/C
C/C H, C
H, C O) to a high degree of oxygenated carbonaceous groups (i.e. C
O) to a high degree of oxygenated carbonaceous groups (i.e. C O, O
O, O C
C O). They reduced DBP formation via structural alternation of the precursor rather than complete organic mitigation, as evidenced by their minor impact in scavenging experiments. Instead, the newly formed Fe(III) characterized by monomeric and polymeric iron, governed the NOM removal process as demonstrated by chelating experiments. In situ Fe(III) facilitated swift coagulation for better charge neutralization with oxidized NOM and ultimately produced more compact flocs with a O
O). They reduced DBP formation via structural alternation of the precursor rather than complete organic mitigation, as evidenced by their minor impact in scavenging experiments. Instead, the newly formed Fe(III) characterized by monomeric and polymeric iron, governed the NOM removal process as demonstrated by chelating experiments. In situ Fe(III) facilitated swift coagulation for better charge neutralization with oxidized NOM and ultimately produced more compact flocs with a O O structure. These characteristics were opposed to conventional Fe(III), which formed loose and elongated flocs. This study elaborates the synergistic roles of in situ oxidation and coagulation in Fe(II)@SBOs systems, offering promising alternatives to conventional coagulation for NOM-DBP mitigation.
O structure. These characteristics were opposed to conventional Fe(III), which formed loose and elongated flocs. This study elaborates the synergistic roles of in situ oxidation and coagulation in Fe(II)@SBOs systems, offering promising alternatives to conventional coagulation for NOM-DBP mitigation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


