一种用于高倍率长循环全固态超高镍阴极的富锂无定形zr基氯化氧固体电解质
IF 9.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
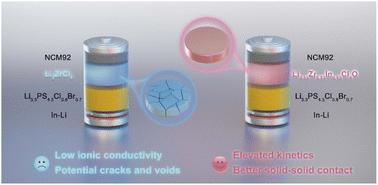
zr基氯化物固体电解质(SE) Li2ZrCl6与4个v级阴极很好地兼容并且具有成本效益,但其低电导率(<1 mS cm−1)会限制电极的容量输送,特别是在高速率下。在Li2+xZr1−xinxcl40o分子式中,我们通过用In3+部分取代Zr4+来调整其Li含量,进一步将zr基氯化氧锂在li2.15 zr0.85 in0.15 cl40o中的室温离子电导率提高到2.11 mS cm−1。冷压后的li2.15 zr0.85 in0.15 cl40在非晶相形成的致密形态下,离子电导率显著提高,显著高于Li2ZrCl6,有利于复合阴极的固-固接触,改善了反应动力学。结果表明,与Li+/Li相比,li2.15 zr0.85 in0.15 cl40和LiNi0.92Co0.03Mn0.05O2的全固态阴极耦合在4C时的容量为175.3 mAh g−1,充电至4.3 V时,450次循环的保留率为91.44%。更吸引人的是,当充电上限增加到4.8 V时,li2.15 zr0.85 in0.15 cl40也使超高镍阴极循环超过250次,容量保持率为81.86%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A Li-enriched amorphous Zr-based oxychloride solid electrolyte for high-rate and long-cycling all-solid-state ultrahigh-nickel cathodes
The Zr-based chloride solid electrolyte (SE) Li2ZrCl6 is well compatible with 4 V-class cathodes and cost-effective, yet its low conductivity (<1 mS cm−1) would restrain the capacity delivery of the electrodes especially at high rates. Herein, we further elevated the room-temperature ionic conductivity of Zr-based lithium oxychloride to 2.11 mS cm−1 in Li2.15Zr0.85In0.15Cl4O by tuning its Li content through partial substitution of Zr4+ with In3+ in the formula Li2+xZr1−xInxCl4O. Cold-pressed Li2.15Zr0.85In0.15Cl4O presents both a dense morphology arising from its amorphous phase and enhanced ionic conductivity, which is significantly higher than that of Li2ZrCl6, facilitating better solid–solid contact and improved reaction kinetics in the composite cathodes. As a result, the all-solid-state cathode coupling Li2.15Zr0.85In0.15Cl4O and LiNi0.92Co0.03Mn0.05O2 shows a capacity of 175.3 mAh g−1 at 4C and a retention of 91.44% for 450 cycles when charged to 4.3 V vs. Li+/Li. More attractively, as the charge upper limit increases to 4.8 V, Li2.15Zr0.85In0.15Cl4O also enables ultrahigh-nickel cathodes to cycle for over 250 cycles with a capacity retention of 81.86%.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


