用于多重DNA甲基化检测的靶刺激纳米粒子组装均匀传感平台的构建
IF 3.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
检测多种肿瘤相关的DNA甲基化变异可以揭示疾病发展的发病机制和生理特征。在此,我们构建了一个目标刺激金纳米颗粒(AuNPs)组装均匀传感平台,用于通过单粒子电感耦合等离子体质谱(SP-ICP-MS)进行多重DNA甲基化检测。目标甲基化DNA的引入可以启动DNA糖基化酶辅助的Linker探针的特异性消化,从而导致aunp聚集的减少。利用SP-ICP-MS可以通过Au脉冲信号的强度和频率很好地区分具有不同大小分布的AuNP聚集体,从而实现甲基化DNA的双模式检测,具有良好的准确性。通过对Linker探针的模块化设计,该同质传感系统可用于多路DNA甲基化分析,具有良好的通用性。以甲基化MLH1和甲基化MGMT为代表,两种定量模式下,甲基化MLH1和甲基化MGMT的定量限分别为25.0 pmol L-1和10.0 pmol L-1。该方法已成功应用于34%的人血清样品中甲基化MLH1和甲基化MGMT的直接分析,证明了该方法对生物基质的强大抗性及其对真实生物样品分析的适用性。均相SP-ICP-MS策略的可编程性和可扩展性使其成为疾病相关生物分子诊断和致病机制研究的一个有希望的候选者。本文章由计算机程序翻译,如有差异,请以英文原文为准。
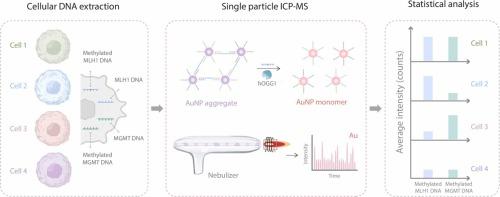
Construction of a target-stimulated nanoparticle-assembly homogeneous sensing platform for multiplexed DNA methylation assay
Detection of multiple tumor-associated DNA methylation variations can shed light on the pathogenesis and physiological characteristics underlying disease development. Herein, we constructed a target-stimulated gold nanoparticles (AuNPs) -assembly homogeneous sensing platform for multiple DNA methylation assays via single-particle inductively coupled plasma mass spectrometry (SP-ICP-MS). The introduction of target methylated DNA could initiate the DNA glycosylase-assisted specific digestion of the Linker probe, thus leading to a reduction in the agglomeration of AuNPs. The AuNP aggregates with varying size distributions can be well discriminated by the intensity and frequency of the Au pulse signal utilizing SP-ICP-MS, thereby enabling dual-mode detection of methylated DNA with good accuracy. Through the modular design of Linker probes, this homogeneous sensing system is feasible for multiplexed DNA methylation analysis, showing good universality. Taking methylated MLH1 and methylated MGMT as representatives, the limit of quantification under the two quantification modes are 25.0 pmol L-1 and 10.0 pmol L-1 for methylated MLH1 and methylated MGMT, respectively. The proposed method was successfully applied to direct analysis of methylated MLH1 and methylated MGMT in 34% human serum samples, demonstrating the robust resistance of the proposed method to biological matrices and its suitability for real biological sample analysis. The programmability and expandability of the homogeneous SP-ICP-MS strategy render it a promising candidate for disease-relevant biomolecule diagnostics and pathogenic mechanism research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sensors and Actuators B: Chemical
工程技术-电化学
CiteScore
14.60
自引率
11.90%
发文量
1776
审稿时长
3.2 months
期刊介绍:
Sensors & Actuators, B: Chemical is an international journal focused on the research and development of chemical transducers. It covers chemical sensors and biosensors, chemical actuators, and analytical microsystems. The journal is interdisciplinary, aiming to publish original works showcasing substantial advancements beyond the current state of the art in these fields, with practical applicability to solving meaningful analytical problems. Review articles are accepted by invitation from an Editor of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


