拉曼光学活性(ROA)法测定多组分手性样品中过量对映体
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
与外消旋药物相比,非异构纯药物具有更好的特异性、更高的治疗指数和优化的药代动力学。然而,制药公司继续生产外消旋药物,除其他外,由于缺乏原位测定光学纯度和绝对构型的工具,这是欧洲药品管理局(EMA)或美国食品药品管理局(FDA)批准原料药所必需的。目前使用的绝对构型和对映体过量测定方法存在明显的局限性(需要单晶、uv活性发色团、手性试剂等)。结果针对药理学行业对手性药物原位有效分析的需求,我们设计并构建了基于拉曼光学活性(ROA)的近线装置,并配备了用于手性化合物原位和流动快速分析的3d打印流动反应器。我们通过测定纯手性样品和多组分手性样品的对映体过量(EE)验证了我们的方法,表明开发的溶液结合了出色的立体灵敏度,测量流动中的手性样品的可能性,以及直接判别手性混合物组分的相当独特的能力。基于偏最小二乘(PLS)回归的定量分析表明,该方法具有很高的准确度和精密度,交叉验证的平均预测误差(RMSECV)不超过1.35% (R2至少为99.95)。据我们所知,我们的工作是第一个成功的流动和原位ROA评价手性混合物中手性纯度的例子。我们的直接方法不需要广泛的样品制备,对映体的初步分离或衍生化。从长远来看,我们的解决方案为operando (In -line) ROA铺平了道路,可以实时监测反应混合物的手性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
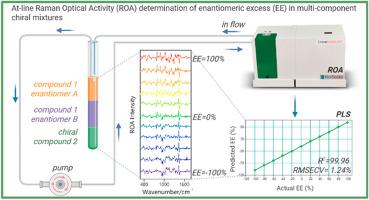

At-line determination of the enantiomeric excess in multi-component chiral samples using Raman optical activity (ROA)
Background
Enantiomerically pure drugs offer better specificity, higher therapeutic index, and optimized pharmacokinetics compared to racemic drugs. However, pharmaceutical companies continue manufacturing racemic medicines, among others, due to the lack of tools for in situ determination of the optical purity and the absolute configuration, which is necessary for approval of drug substance by the European Medicines Agency (EMA) or the US Food and Drug Administration (FDA). Currently used methods for the absolute configuration and the enantiomeric excess determination have significant limitations (require single crystal, UV-active chromophores, chiral reagents, etc).
Results
Addressing this need of pharmacological industry for the effective analysis of chiral drugs in situ in solution, we have designed and constructed the at-line setup based on Raman optical activity (ROA) and equipped with the 3D-printed flow reactor dedicated for fast in situ and in-flow analysis of chiral compounds. We have validated our approach by the determination of the enantiomeric excess (EE) of pure and multi-component chiral samples showing that the developed solution combines the excellent stereosensitivity, possibility to measure chiral samples in-flow, and a rather unique capability of direct discrimination of components of chiral mixtures. The quantitative analysis based on Partial Least Squares (PLS) regression has demonstrated that the accuracy and precision of the method is very high with the average prediction error from cross-validation (RMSECV) not exceeding 1.35 % (for R2 at least 99.95) for ROA spectra recorded in 20 min.
Significance
According to our best knowledge, our work is a first example of successful in-flow and in situ ROA evaluation of chiral purity in chiral mixtures. Our direct approach does not require extensive sample preparation, preliminary separation of enantiomers or derivatization. In the long-term, our solution paves the way toward operando (in-line) ROA, enabling real-time monitoring of chirality in reaction mixtures.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


