Y502-2304的发现,一种有效的c-Myc g -四重稳定剂,用于治疗多发性骨髓瘤
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
c-Myc是一种原癌基因,在包括多发性骨髓瘤(MM)在内的各种癌症中经常过表达,并与不良临床结果相关。由于缺乏明确的小分子结合口袋,直接靶向c-Myc仍然是一个艰巨的挑战。在c-Myc启动子的NHE III1区域形成的g -四重体(G4)提供了抑制c-Myc转录的另一种策略。鉴于噻吩[3,2-e][1,2,4]三唑[1,5-c]嘧啶的结构特征与G4稳定剂一致,我们寻求基于该支架鉴定新的化合物。基于结构相似性的ChemDiv小分子文库虚拟筛选确定了23个衍生物,优先进行进一步研究。其中,化合物Y502-2304在MM细胞中表现出选择性稳定c-Myc G4结构和有效的抗增殖活性。此外,Y502-2304剂量依赖性地下调了c-Myc mRNA和蛋白的表达,而对其他含有g4的癌基因的影响很小。在机制上,Y502-2304诱导MM细胞凋亡,其特征是γ - h2ax水平升高、活性氧(ROS)生成增加和线粒体功能障碍。它还激活了p53通路,上调了下游促凋亡蛋白Noxa和PUMA。Y502-2304在异种移植MM模型中显著抑制肿瘤生长,而在体内没有引起明显的毒性。这些发现强调Y502-2304是一种选择性的c-Myc G4稳定剂,具有很强的治疗MM的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
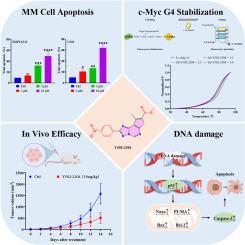
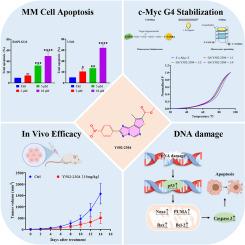
Discovery of Y502-2304, a potent c-Myc G-quadruplex stabilizer for the treatment of multiple myeloma
c-Myc is a proto-oncogene that is frequently overexpressed in various cancers, including multiple myeloma (MM), and is associated with poor clinical outcomes. Due to the lack of well-defined small-molecule binding pockets, directly targeting c-Myc remains a formidable challenge. The G-quadruplex (G4) formed in the NHE III1 region of the c-Myc promoter provides an alternative strategy to suppress c-Myc transcription. Given the structural features of thieno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine that are consistent with G4 stabilizers, we sought to identify novel compounds based on this scaffold. A structural similarity-based virtual screen of the ChemDiv small-molecule library identified 23 of its derivatives, which were prioritized for further investigation. Among these, compound Y502–2304 demonstrated selective stabilization of the c-Myc G4 structure and potent antiproliferative activity in MM cells. Moreover, Y502–2304 dose-dependently downregulated c-Myc mRNA and protein expression while exerting minimal effects on other G4-containing oncogenes. Mechanistically, Y502–2304 induced apoptosis in MM cells, characterized by elevated γH2AX levels, increased reactive oxygen species (ROS) generation, and mitochondrial dysfunction. It also activated the p53 pathway and upregulated the downstream pro-apoptotic proteins Noxa and PUMA. Y502–2304 significantly inhibited tumor growth in a xenograft MM model without inducing notable toxicity in vivo. These findings underscore Y502–2304 as a selective c-Myc G4 stabilizer with strong therapeutic potential for MM.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


