补骨脂联合核桃核通过Nrf2/GPX4/SLC7A11途径抑制铁下垂,改善绝经后骨质疏松症。
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
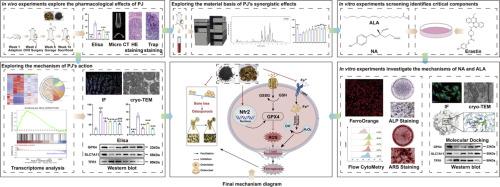
背景:绝经后骨质疏松症(PMOP)是一种由雌激素(E2)缺乏引起的骨代谢紊乱。中药补骨脂(P)常与核桃仁(J, Juglans regia L.)合用治疗骨质疏松症。然而,核桃仁与补骨脂(PJ)联合使用是否比单独使用补骨脂更有效尚不清楚,其协同作用的物质基础和机制也不完全清楚。目的:本研究旨在阐明PJ治疗PMOP的协同作用机制,并鉴定其有效成分及其靶点。方法:采用超高效液相色谱-质谱联用技术对PJ中的化合物进行分析。采用双侧卵巢切除术(OVX)建立大鼠PMOP模型。采用血清ELISA、micro-CT、H&E、TRAP染色评价股骨病理变化。UPLC-MS/MS分析也用于筛选血清中的活性成分,重点关注关键单体。转录组测序鉴定核心通路。用erastin处理建立MC3T3-E1型铁吊模型。采用组合指数评价了各单体的协同效应。除了测量细胞活力外,还使用低温透射电镜、共聚焦激光扫描显微镜和流式细胞术验证了关键单体对铁凋亡和成骨细胞分化的影响。通过分子对接、分子动力学(MD)模拟、微尺度热泳(MST)、免疫荧光和western blotting来验证该活性的靶点。结果:体内实验结果表明,PJ对ovx所致骨质疏松的改善作用比P单用更显著。UPLC-MS/MS结果显示,与j相容性后,8种潜在活性成分的血清浓度显著升高。转录组分析显示,OVX组中ferroptosis显著富集,而体内实验表明,PJ通过调节Nrf2/GPX4/SLC7A11途径抑制ferroptosis。体外实验鉴定去甲荞麦酸(NA)和α-亚麻酸(ALA)是PJ的主要药效学成分,证实两种成分协同抑制铁下垂,同时促进成骨矿化。这种作用依赖于Nrf2/GPX4/SLC7A11通路的激活。MST和MD模拟显示NA和ALA都直接与Keap1结合,从而促进Nrf2核易位并引发下游生物反应。此外,当ML385体外抑制Nrf2表达时,NA和ALA对铁下垂的抑制作用被抑制。结论:本研究首次系统证明核桃仁可通过提高补骨脂有效成分的生物利用度和形成NA和ALA的协同网络来增强补骨脂对ppu的治疗作用。此外,Nrf2/GPX4/SLC7A11通路被确定为NA和ALA协同抑制成骨细胞铁凋亡的主要机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Psoraleae Fructus combined with Walnut kernels improves postmenopausal osteoporosis by inhibiting ferroptosis through the Nrf2/GPX4/SLC7A11 pathway
Background
Postmenopausal osteoporosis (PMOP) is a bone metabolic disorder caused by estrogen (E2) deficiency. The traditional Chinese medicine Psoraleae Fructus (P) is often used in combination with walnut kernels (J, Juglans regia L.) to treat osteoporosis. However, whether the combination of walnut kernels and Psoraleae Fructus (PJ) is more effective than Psoraleae Fructus alone remains unclear, and the material basis and mechanism of the synergistic effects of this combination are not fully understood.
Purpose
This study aimed to elucidate the synergistic mechanisms of PJ in the treatment of PMOP and to identify the active components and their targets.
Methods
The compounds in PJ were analyzed using UPLC-MS/MS. Bilateral ovariectomy (OVX) was performed to establish a rat model of PMOP. Femoral pathological changes were evaluated by serum ELISA, micro-CT, H&E and TRAP staining. UPLC-MS/MS analysis was also performed to screen for active components in the serum, focusing on key monomers. Transcriptome sequencing was conducted to identify core pathways. An MC3T3-E1 ferroptosis model was established by erastin treatment. A combination index was used to evaluate the synergistic effects of the monomers. In addition to measuring cell viability, the effects of the key monomers on ferroptosis and osteoblastic differentiation were verified using cryogenic transmission electron microscopy, confocal laser scanning microscopy, and flow cytometry. Molecular docking, molecular dynamics (MD) simulations, microscale thermophoresis (MST), immunofluorescence, and western blotting were performed to validate the targets of this activity.
Results
In vivo experimental results indicated that PJ significantly improved OVX-induced osteoporosis more prominently than P alone. The UPLC-MS/MS results showed that the serum concentrations of eight potentially active components increased significantly after compatibility with J. Transcriptome analysis revealed significant enrichment of ferroptosis in the OVX group, whereas in vivo experiments demonstrated that PJ inhibited ferroptosis by regulating the Nrf2/GPX4/SLC7A11 pathway. In vitro experiments identified norbakuchinic acid (NA) and α-linolenic acid (ALA) as the main pharmacodynamic components of PJ and confirmed that both components synergistically inhibited ferroptosis while promoting osteogenic mineralization. This effect was dependent on Nrf2/GPX4/SLC7A11 pathway activation. MST and MD simulations revealed that both NA and ALA were directly bind to Keap1, thereby promoting Nrf2 nuclear translocation and triggering downstream biological responses. Furthermore, when Nrf2 expression was inhibited in vitro by ML385, the inhibitory effects of NA and ALA on ferroptosis were suppressed.
Conclusion
This study provided the first systematic evidence that walnut kernels can enhance the therapeutic efficacy of Psoraleae Fructus against PMOP by improving the bioavailability of active constituents and forming a synergistic network of NA and ALA. Furthermore, it has been determined that the Nrf2/GPX4/SLC7A11 pathway serves as the principal mechanism through which NA and ALA synergistically inhibit osteoblast ferroptosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


