naio4促进胺与h -膦氧化物的磷酸化,在水中构建P(O)-N键。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们发现了一种简便的、绿色的P(O)-N键形成方案,即在水中,由naio4引发的稳定的、容易获得的胺与次级氧化膦的磷酸化。该方案具有良好的官能团耐受性和广泛的衬底范围,不需要金属或碱添加剂,为构建P(O)-N键提供了另一种方法。机理研究表明,二次氧化膦的磷酸化过程促进了水中胺的磷酸化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
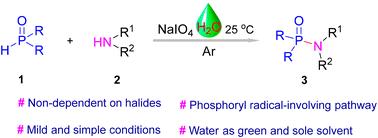
NaIO4-promoted phosphorylation of amines with H-phosphine oxides to construct P(O)–N bonds in water
A facile and green protocol for P(O)–N bond formation was discovered through NaIO4-initiated phosphorylation of stable and readily available amines with secondary phosphine oxides in water. This protocol shows good functional group tolerance and broad substrate scope without metal or base additives, providing an alternative method for constructing P(O)–N bonds. Mechanistic studies suggested that a phosphoryl radical-involving process from secondary phosphine oxides facilitated the phosphorylation of amines in water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


