动脉粥样硬化斑块的多模式景观:质谱成像的空间组学方法
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
动脉粥样硬化斑块是一种复杂的异质结构,起源于血管中的脂肪条纹,由脂质和泡沫细胞的积累形成。随着时间的推移,这些病变发展为炎症、平滑肌细胞增殖和表型转换,细胞外基质沉积促进斑块生长,最终导致斑块断裂、反应性血栓形成和心血管事件,如心肌梗死和中风。传统的质谱(MS)分析已经对斑块形成和疾病进展的分子机制产生了重要的见解,但它无法确定病变内的空间异质性和微环境复杂性。基于质谱成像(MSI)的组学(包括空间脂质组学、蛋白质组学和代谢组学)的最新进展,使动脉粥样硬化斑块中分子分布在细胞分辨率上的可视化成为可能。这些技术有望阐明不同的细胞串扰、病变易损性和性别特异性疾病机制,这些机制有助于斑块的发展和破裂。本文综述了基于ms的空间组学的最新进展及其在实验模型和人类样本中对动脉粥样硬化斑块的应用。我们重点介绍了最近的发现,探讨了它们对精准医学和转化研究的影响,并讨论了当前在样品制备和数据整合方面的挑战。尽管面临挑战,我们建议使用人工智能(AI)集成基于ms的空间组学,以增强数据集成、解释和转化应用于动脉粥样硬化研究。这些进展有望拓宽我们对动脉粥样硬化的理解,并确定新的治疗靶点,以限制心血管疾病的负担。本文章由计算机程序翻译,如有差异,请以英文原文为准。
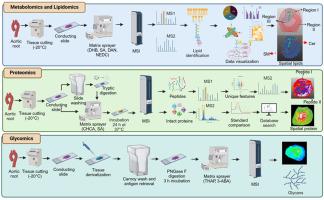
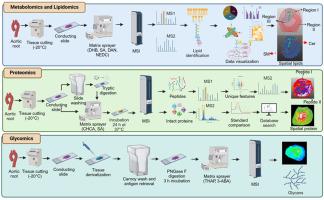
Multimodal landscape of atherosclerotic plaques: A spatial omics approach with mass spectrometry imaging
Atherosclerotic plaques are complex and heterogeneous structures, originating as fatty streaks in the vasculature and formed by the accumulation of lipids and foam cells. Over time, these lesions progress as inflammation, smooth muscle cell proliferation and phenotypic switching, and extracellular matrix deposition contribute to plaque growth, culminating in their fracture, reactive thrombogenesis, and a cardiovascular event such as myocardial infarction and stroke. Traditional bulk mass spectrometry (MS) analysis has yielded critical insights into the molecular mechanisms of plaque formation and disease progression, but it is unable to determine the spatial heterogeneity and microenvironmental complexity within the lesion. Recent advances in mass spectrometry imaging (MSI) based omics, including spatial lipidomics, proteomics, and metabolomics, have enabled unprecedented visualization of molecular distribution in atherosclerotic plaques at cellular resolution. These techniques promise to elucidate the distinct cellular crosstalk, lesion vulnerability, and sex-specific disease mechanisms that contribute to plaque development and rupture. This review examines the recent advances in MS-based spatial omics and their application to atherosclerotic plaques in both experimental models and human samples. We highlight recent findings, explore their implications for precision medicine and translational research, and discuss current challenges in sample preparation and data integration. Despite challenges, we suggest approaches for integration of MS-based spatial omics using artificial intelligence (AI) to enhance data integration, interpretation, and translational applications in atherosclerosis research. These advances promise to broaden our understanding of atherosclerosis and identify novel therapeutic targets to limit the burden of cardiovascular disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


