镍钴双金属催化剂上5-羟甲基糠醛加氢脱氧制2,5-二甲基呋喃的优化及反应动力学
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
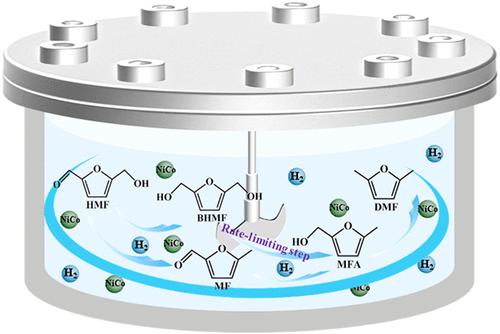
2,5-二甲基呋喃(DMF)是一种很有前途的液体燃料,通过5-羟甲基呋喃(HMF)的加氢脱氧(HDO)选择性生产DMF对于可持续生物质升级至关重要。本研究采用沉积-沉淀法制备了负载型双金属Ni60Co40/SiO2催化剂和参考型Ni100/SiO2催化剂,优化了HDO反应条件(温度180 ~ 210℃,氢气压力1 ~ 3 MPa, HMF浓度0.15 ~ 0.3 mol/L)。结果表明,Ni60Co40/SiO2催化剂在最佳条件下(200℃,2 MPa H2压力,0.23 mol/L HMF, 180 min)实现了完全的HMF转化,DMF收率为95%,超过了Ni100/SiO2催化剂(80% DMF收率)。建立了HMF转化的动力学模型,揭示了一级反应动力学。Ni60Co40/SiO2的表观活化能(Ea)为41.6 kJ/mol,低于Ni100/SiO2的60.2 kJ/mol。定量分析表明,2,5-二(羟甲基)呋喃(BHMF)氢解制5-甲基呋喃醇(MFA)是反应的限速步骤。模型与实验数据吻合良好(R > 0.96)。此外,Ni60Co40/SiO2催化剂在5个反应循环中比Ni100/SiO2催化剂更稳定。详细分析了稳定性的差异。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Optimization and Reaction Kinetics of Hydrodeoxygenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran over Ni–Co Bimetallic Catalysts
Selective production of 2,5-dimethylfuran (DMF), a promising liquid fuel, via hydrodeoxygenation (HDO) of 5-hydroxymethylfurfural (HMF) is crucial for sustainable biomass upgrading. In this study, a supported bimetallic Ni60Co40/SiO2 catalyst and a reference Ni100/SiO2 catalyst, prepared via deposition–precipitation, were used to optimize HDO reaction conditions (temperature, 180–210 °C; hydrogen pressure, 1–3 MPa; HMF concentration, 0.15–0.3 mol/L). Results showed that the Ni60Co40/SiO2 catalyst achieved complete HMF conversion with a 95% DMF yield under optimal conditions (200 °C, 2 MPa H2 pressure, 0.23 mol/L HMF, and 180 min), surpassing the Ni100/SiO2 catalyst (80% DMF yield). A kinetic model for HMF conversion was developed, revealing first-order reaction kinetics. The apparent activation energy (Ea) for Ni60Co40/SiO2 was 41.6 kJ/mol, which is lower than that for Ni100/SiO2 (60.2 kJ/mol). Quantitative analysis of the reaction pathways showed that hydrogenolysis of 2,5-bis(hydroxymethyl)furan (BHMF) to 5-methylfurfuryl alcohol (MFA) is the rate-limiting step. The model achieved excellent agreement with experimental data (R > 0.96). Moreover, the Ni60Co40/SiO2 catalyst was more stable than the Ni100/SiO2 catalyst over five reaction cycles. The difference in stability was analyzed in detail.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


