解读阿尔茨海默病大脑中的三维生物分子分布:一种结合免疫组织化学和MALDI MS成像的多组学方法
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
阿尔茨海默病(AD)的特点是大脑中复杂而独特的空间和分子变化,这些变化有助于其发病和进展,包括蛋白质、脂质和神经炎症途径的破坏。在这项研究中,我们引入了一种三维(3D)空间多组学成像方法,该方法将脂质谱成像(MSI)与使用10-plex光可切割质量标签(PC-MT)探针的靶向蛋白质组学分析相结合。野生型(WT)和AD模型小鼠的脑组织在四个冠状水平切片,间隔1500 μm,能够重建深度分辨率的分子图谱。脂质MSI揭示了AD和WT大脑中磷脂酰胆碱(PC)、磷脂酰乙醇胺(PE)和其他脂质类别的区域和深度特异性改变。同时,使用PC-MT探针的蛋白MSI鉴定了不同深度的皮层、海马和丘脑的神经炎症和神经退行性标记物的空间差异变化。这个整合平台揭示了AD病理特有的脂质失调和蛋白质过表达的共定位模式,突出了易感的脑亚区。我们的研究结果表明,在3D中结合靶向蛋白和非靶向脂质成像可以发现神经退行性疾病中空间协调的分子特征,为生物标志物的发现和治疗开发提供了一个强大的框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
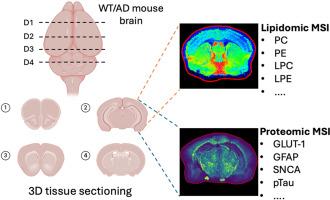
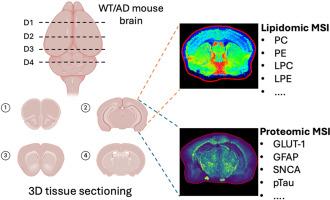
Deciphering the three-dimensional biomolecular distribution in the Alzheimer's disease brain: A multiomic approach integrating immunohistochemistry with MALDI MS imaging
Alzheimer's disease (AD) is characterized by complex and distinct spatial and molecular changes in the brain that contribute to its onset and progression, which includes disruptions in proteins, lipids, and neuroinflammatory pathways. In this study, we introduce a three-dimensional (3D) spatial multi-omics imaging approach that integrates lipid mass spectrometry imaging (MSI) with targeted proteomic analysis using 10-plex photo-cleavable mass tag (PC-MT) probes. Brain tissues from wild-type (WT) and AD model mice were sectioned at four coronal levels, spaced 1500 μm apart, enabling the reconstruction of a depth-resolved molecular atlas. Lipid MSI revealed region- and depth-specific alterations in phosphatidylcholine (PC), phosphatidylethanolamine (PE), and other lipid classes between AD and WT brains. In parallel, protein MSI using PC-MT probes identified spatially distinct changes in neuroinflammatory and neurodegenerative markers across the cortex, hippocampus, and thalamus at different depths. This integrative platform uncovered co-localization patterns of lipid dysregulation and protein overexpression unique to AD pathology, highlighting vulnerable brain subregions. Our findings demonstrate that combining targeted protein and untargeted lipid imaging in 3D enables the discovery of spatially coordinated molecular signatures in neurodegeneration, offering a powerful framework for biomarker discovery and therapeutic development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


