双酚A通过氧化应激损害小鼠囊胚发育潜能
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
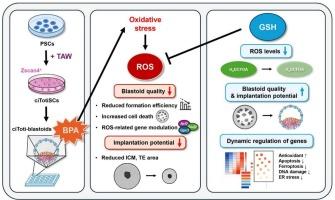
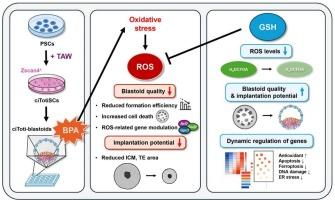
双酚A (BPA)是一种广泛存在的环境内分泌干扰物,对胚胎发育和着床有不利影响。传统的生殖毒性试验通常依赖于动物来源的配子或胚胎。然而,这些方法是侵入性的,在评估早期种系发育方面能力有限。为了解决这些局限性,我们使用了来自多能干细胞(PSCs)的合成胚胎模型来评估bpa诱导的胚胎毒性,而不使用实际的卵母细胞或胚胎。小鼠PSCs通过一种确定的小分子组合转化为化学诱导的全能样干细胞(citotisc)。这些citotisc通过倒置金字塔型微孔板(AggreWell系统)的三维聚集产生囊胚(ciToti-blastoids)并培养5 d。为了研究环境有毒物质对囊胚形成的影响,在囊胚形成过程中暴露于5 μM BPA。5 μM BPA处理后,囊胚形成效率降至约14% %,而对照组约为73% %。为了评估植入潜力,将已建立的囊胚进一步培养在matrigel包被的培养皿上。bpa处理的囊胚显示出内细胞团(ICM)面积和滋养外胚层(TE)面积分别减少约96% %和72% %,表明着床潜力受损。BPA的这些有害影响伴随着citoanti -blastoid形成过程中活性氧(ROS)水平的升高。与抗氧化剂谷胱甘肽(GSH; 0.5 mM)(一种ROS清除剂)共同处理,恢复了囊胚形成效率(约54 %),并导致与对照组相似的标准化ICM和TE区域。转录组学分析显示,BPA诱导内质网应激、DNA损伤和细胞凋亡,而GSH处理可减轻这些损伤。这些结果强调了氧化应激在bpa诱导的胚胎毒性中的作用,并支持干细胞衍生的囊胚模型作为评估环境化学品生殖毒性的无卵平台的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Bisphenol A impairs developmental potential of mouse blastoids through oxidative stress
Bisphenol A (BPA) is a widely encountered environmental endocrine disruptor with detrimental effects on embryonic development and implantation. Conventional reproductive toxicity tests often rely on animal-derived gametes or embryos. However, these approaches are invasive and limited in their ability to assess early germline development. To address these limitations, we used a synthetic embryo model derived from pluripotent stem cells (PSCs) to evaluate BPA-induced embryotoxicity without using actual oocytes or embryos. Mouse PSCs were converted into chemically induced totipotent-like stem cells (ciTotiSCs) using a defined small-molecule combination. These ciTotiSCs generated blastoids (ciToti-blastoids) through three-dimensional aggregation in inverted pyramid-shaped microwell plates (AggreWell system) and cultured for 5 d. To investigate the impact of environmental toxicants on early embryogenesis, blastoids were exposed to 5 μM BPA during formation. Blastoid formation efficiency was reduced to approximately 14 % following treatment with 5 μM BPA, compared with approximately 73 % in the control. To assess implantation potential, established blastoids were further cultured on the Matrigel-coated dish. BPA-treated blastoids exhibited a reduction in inner cell mass (ICM) area and in trophectoderm (TE) area, approximately 96 % and 72 %, respectively, indicating impaired implantation potential. These detrimental effects of BPA were accompanied by elevated reactive oxygen species (ROS) levels during ciToti-blastoid formation. Co-treatment with the antioxidant glutathione (GSH; 0.5 mM), a ROS scavenger, restored blastoid formation efficiency (approximately 54 %) and resulted in a similar normalized ICM and TE areas comparable to control. Transcriptomic analysis revealed that BPA induced endoplasmic reticulum stress, DNA damage, and apoptosis, all of which were mitigated by GSH treatment. These results highlight the role of oxidative stress in BPA-induced embryotoxicity and support the utility of stem cell-derived blastoid models as egg-free platforms for assessing the reproductive toxicity of environmental chemicals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


