解除超级细菌:抑制NDM-1和其他临床相关金属β-内酰胺酶的新领域
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
金属β-内酰胺酶(MBLs)的出现和全球传播,特别是新德里金属β- β-内酰胺酶(NDM)、维罗纳整合子编码的金属β- β-内酰胺酶(VIM)和亚胺培烯酶(IMP),对β-内酰胺类抗生素(包括碳青霉烯类抗生素)的疗效构成重大威胁,这些抗生素通常被视为抵御多重耐药细菌感染的最后一道防线。本文综合分析了针对NDM、VIM和IMP酶的MBL抑制剂(MBLIs)的最新进展,特别关注NDM-1。这些抑制剂根据负责其活性的关键官能团进行分类,从而深入了解控制其效力和选择性的结构活性关系(SAR)。我们强调具有有效抑制数据(IC50/Ki值)的代表性化合物,通过分子对接研究和与目标mls可用的共晶结构来阐明它们的结合相互作用。还讨论了这些抑制剂的合成方法。这篇综述的目的是提供一个详细的,有功能组织的框架,为正在进行的设计有效的,广谱的MBL抑制剂,能够恢复β-内酰胺抗生素的效用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
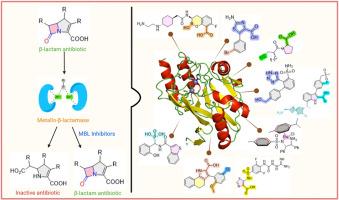
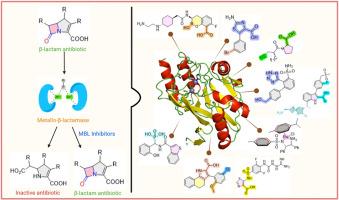
Disarming superbugs: New frontiers in inhibiting NDM-1 and other clinically relevant metallo-β-lactamases
The emergence and global spread of Metallo-β-lactamases (MBLs), particularly New Delhi Metallo-β-lactamase (NDM), Verona Integron-encoded Metallo-β-lactamase (VIM), and Imipenemase (IMP), pose a significant threat to the efficacy of β-lactam antibiotics, including carbapenems, often regarded as the last line of defence against multidrug-resistant bacterial infections. This review comprehensively analyzes recent advances in the development of MBL inhibitors (MBLIs), targeting NDM, VIM, and IMP enzymes with a special focus on NDM-1. The inhibitors are categorized based on key functional groups responsible for their activity, offering insight into the structure activity relationships (SAR) that govern their potency and selectivity. We highlight representative compounds with potent inhibition data (IC50/Ki values), supported by molecular docking studies and where available co-crystal structures with target MBLs to elucidate their binding interactions. Synthetic approaches for these inhibitors are also discussed. This review aims to provide a detailed, functionally organized framework for ongoing efforts in designing potent, broad-spectrum MBL inhibitors capable of restoring the utility of β-lactam antibiotics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


