价和亲和度决定了抗tnfr2纳米体融合蛋白的激动活性
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
我们发现了几个tnfr2特异性纳米体(Nbs)。当形成二聚体Fc融合蛋白时,这些纳米体没有表现出激动活性。为了提高活性,我们将tnfr2特异性Nb:188的一个、两个或三个拷贝基因融合到IgG1抗体、Fab片段、Fc结构域或tenascin-C的三聚结构域的恒定区域,从而构建出2-12个Nb:188结构域。具有2或3个Nb:188结构域的结构体没有活性或活性极低,而具有4和5个Nb:188结构域的结构体具有中等活性。然而,具有6个或更多Nb:188结构域的结构体表现出强烈的激动作用,在低皮摩尔浓度范围内达到最大TNFR2激活的一半。同样,与其他tnfr2特异性Nb结构域产生的六聚体结构也表现出强大的激动作用。对各种其他基于配体和抗体的TNFR2激动剂的基准测试显示,六美体3xNb:188-Fc格式具有优越的特异性活性,并有效地扩展调节性T细胞(Tregs)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
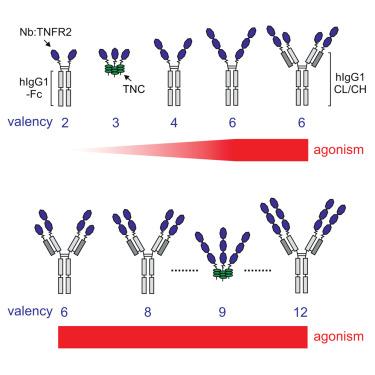
Valence and avidity determine the agonistic activity of anti-TNFR2 nanobody fusion proteins
We identified several TNFR2-specific nanobodies (Nbs). When formatted as dimeric Fc fusion proteins, these nanobodies exhibited no agonistic activity. To improve activity, we genetically fused one, two, or three copies of the TNFR2-specific Nb:188 to the constant regions of an IgG1 antibody, an Fab fragment, an Fc domain or the trimerization domain of tenascin-C resulting in constructs with 2–12 Nb:188 domains. Constructs with 2 or 3 Nb:188 domains displayed no or minimal activity, while those with 4 and 5 Nb:188 domains demonstrated moderate activity. However, constructs with 6 or more Nb:188 domains exhibited potent agonism, reaching half-maximal TNFR2 activation at concentrations in the low picomolar range. Similarly, hexameric constructs generated with other TNFR2-specific Nb domains demonstrated robust agonism, too. Benchmarking against various other ligand- and antibody-based TNFR2 agonists revealed that the hexameric 3xNb:188-Fc format displays superior specific activity and efficiently expands regulatory T cells (Tregs).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


