在低温电镜条件下,脂质调节RyR1的打开概率
IF 4.3
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
Ryanodine受体(RyRs)是细胞内的四聚体离子通道,负责从肌浆网和内质网释放Ca2+。Ryanodine receptor 1 (RyR1)异构体对肌肉收缩至关重要,研究最为广泛。虽然冷冻电子显微镜(cryo-EM)已经有助于揭示RyR门控机制的近原子细节,但在冷冻电子显微镜条件下RyR1的打开概率明显低于电生理研究中观察到的。在这里,我们提出了一项低温电镜研究,研究了RyR1在不同脂质浓度的CHAPS中溶解的打开概率。我们发现,当脂质浓度从0.001%增加到0.05%时,RyR1的打开概率从16%提高到84%,而重组成脂质纳米盘的RyR1仍保持关闭状态。我们在最高脂质浓度下重建的图谱中模拟了72个脂质分子。这些发现表明,在低温电镜条件下,脂质在调节溶解的RyR1通道的开放部分中发挥了重要作用,并为RyR1门控的结构研究提供了最佳的脂质模拟物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
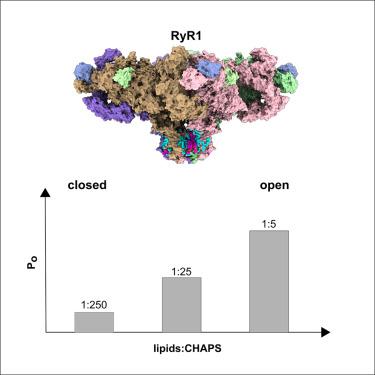
Lipids modulate the open probability of RyR1 under cryo-EM conditions
Ryanodine receptors (RyRs) are intracellular tetrameric ion channels responsible for Ca2+ release from the sarcoplasmic and endoplasmic reticulum. Ryanodine receptor 1 (RyR1) isoform, critical for muscle contraction, has been studied most extensively. While cryoelectron microscopy (cryo-EM) has been instrumental in revealing near-atomic details of RyR gating mechanisms, the open probability of RyR1 under cryo-EM conditions is notably lower than that observed in electrophysiological studies. Here, we present a cryo-EM study examining the open probability of RyR1 solubilized in CHAPS with varying lipid concentrations. We found that increasing lipid concentration from 0.001% to 0.05% raised the RyR1 open probability from 16% to 84%, whereas RyR1 reconstituted into lipid nanodiscs remained closed. We modeled 72 lipid molecules in the map reconstructed at the highest lipid concentration. These findings demonstrate the important role of lipids in modulating the open fraction of solubilized RyR1 channels under cryo-EM conditions and suggest optimal lipid mimetics for structural studies of RyR1 gating.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


