亲脂性整合生物正交自催化一氧化氮供体-铂(IV)前药增强食管癌抗肿瘤疗效
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
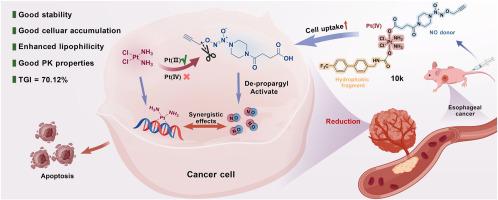
食管癌(EC)是一种全球普遍存在的恶性肿瘤,有效的治疗靶点有限,其中顺铂(CDDP)/5-氟尿嘧啶(5-FU)联合治疗仍然是标准的化疗支柱。一氧化氮(NO)和铂(IV)配合物在抗肿瘤治疗方面引起了很大的研究兴趣。先前设计的整合前药细胞摄取不良。在此,我们报道了一系列新的亲脂性整合生物正交自催化NO供体/Pt(IV)前药的设计、合成和生物学评价,其中细胞质还原剂催化Pt(Ⅱ)和NO的释放,在癌细胞中产生协同抗肿瘤作用。体外生物学研究显示,10k对食管癌细胞系的IC50值比CDDP低1.3- 4.4倍,与显著增强的亲脂性相关。复合物10k轴向位置的亲脂修饰显著增强了KYSE-520细胞的Pt积累和DNA铂化,分别是CDDP的10.4倍和4.7倍。首选配合物10k表现出良好的稳定性和药代动力学性质。与CDDP相比,10k在体内表现出强大的抗食管癌作用(对KYSE-520异种移植模型有71.1%的肿瘤生长抑制(TGI)),并抑制肺转移,无明显的全身毒性。综上所述,综合前药10k是一种很有前景的治疗EC的候选药物,值得进一步深入研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Lipophilic Integrated Bioorthogonal Self-Catalyzed Nitric Oxide Donor–Platinum(IV) Prodrugs for Enhanced Antitumor Efficacy Against Esophageal Cancer
Esophageal cancer (EC) represents a globally prevalent malignancy with limited validated therapeutic targets, for which the cisplatin (CDDP)/5-fluorouracil (5-FU) combination remains the standard chemotherapy backbone. Nitric oxide (NO) and platinum(IV) complexes have attracted significant research interest in antitumor therapy. Previously designed integrated prodrugs suffered from poor cellular uptake. Herein, we reported the design, synthesis, and biological evaluation of a series of novel lipophilic integrated bioorthogonal self-catalyzed NO donor/Pt(IV) prodrugs, in which cytoplasmic reductants catalyze the release of Pt(Ⅱ) and NO to produce synergistic antitumor effects in cancer cells. In vitro biological studies revealed that 10k exhibited 1.3- to 4.4-fold lower IC50 values than CDDP against esophageal cancer cell lines, correlating with significantly enhanced lipophilicity. The lipophilic modification at the axial position of complex 10k significantly enhanced cellular Pt accumulation and DNA platination in KYSE-520 cells by 10.4- and 4.7-fold, respectively, compared to CDDP. The preferred complex 10k demonstrated favorable stability and pharmacokinetic properties. Compared with CDDP, 10k exhibited potent in vivo anti-esophageal cancer effects (71.1% tumor growth inhibition (TGI) against the KYSE-520 xenograft model) as well as inhibited lung metastasis, without significant systemic toxicity. Overall, the integrated prodrug 10k represents a promising candidate for EC treatment and deserves further in-depth studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


