光催化甲烷氧化中钯的组成和分散控制选择性
IF 9.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
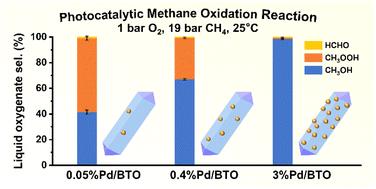
由于甲烷的高键解离能和难以实现高选择性,在温和条件下甲烷选择性氧化制甲醇面临很大挑战。本研究考察了钯的组成和分散对二氧化钛纳米棒(BTO)上甲烷光催化氧化的影响。在可见光到中红外(IR)范围内的原位时间分辨瞬态吸收(TA)光谱以及甲烷氧化前后的x射线光电子能谱(XPS)分析表明,高Pd负载和低Pd色散的BTO纳米棒在光照下在Pd/PdO纳米颗粒中表现出明显的电子俘获。这种电子俘获促进了PdO的自还原成金属Pd (Pd0),进而促进了CH3OH的选择性生成,选择性为98%。相比之下,适度的Pd负载和分散在BTO纳米棒上,增强了电子向O2的转移,减少了电子捕获,并导致更高浓度的混合Pd0/PdO纳米颗粒,有利于初级氧合物- CH3OOH和CH3OH的形成。DFT计算和实验结果表明,Pd0有利于O2直接三电子还原为*OH或˙OH,并与*CH3或˙CH3偶联生成CH3OH。同时PdO促进O2单电子还原为*OOH或˙OOH,生成CH3OOH。这项工作为设计用于选择性甲烷氧化的高效光催化剂提供了有价值的见解,并强调了Pd的组成和分散在调节甲烷中的电荷动力学和控制产物选择性方面的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Pd composition and dispersion control selectivity in photocatalytic methane oxidation
Selective methane oxidation to methanol under mild conditions presented a significant challenge due to the high bond dissociation energy of methane and the difficulty in achieving high selectivity. This study investigated how Pd composition and dispersion influenced photocatalytic oxidation of methane over brookite TiO2 (BTO) nanorods, using molecular O2 and water as oxidants. In situ time-resolved transient absorption (TA) spectroscopy in the visible to mid-infrared (IR) range, along with X-ray photoelectron spectroscopy (XPS) analysis before and after methane oxidation, revealed that BTO nanorods with high Pd loading and lower Pd dispersion exhibited significant electron trapping in the Pd/PdO nanoparticles under light irradiation. This electron trapping promoted the self-reduction of PdO to metallic Pd (Pd0), which in turn drove the selective production of CH3OH with 98% selectivity. In contrast, a moderate Pd loading and dispersion on the BTO nanorods enhanced electron transfer to O2, reducing electron trapping, and resulted in a higher concentrations of mixed Pd0/PdO nanoparticles, favoring the formation of primary oxygenates – CH3OOH and CH3OH. DFT calculations and experimental findings showed that Pd0 facilitates the direct three-electron reduction of O2 to *OH or ˙OH, which subsequently coupled with *CH3 or ˙CH3 to form CH3OH. Meanwhile PdO promotes the one-electron reduction of O2 to *OOH or ˙OOH, leading to CH3OOH formation. This work provides valuable insights into the design of efficient photocatalysts for selective methane oxidation and underscores the critical role of Pd composition and dispersion in modulating charge dynamics and steering product selectivity in methane.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


