广藿香倍半萜类化合物的分离、表征及抗神经炎活性研究。
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
对大藿香进行植物化学鉴定,分离鉴定出16种倍半萜类化合物,其中尤达木烷型(1-6、9和11)、双abolane型(8和10)、卡萝烷型(13-14)、愈创木烷型(15-16)和其他类型(7和12)。其中,化合物1-10,即pogostesquines A-J,是以前描述过的10种化合物。化合物7具有一个不寻常的主链,在C-8取代了异丙基而不是C-7。通过广泛的光谱分析、单晶x射线衍射分析和ECD计算证实了它们的结构。所有化合物均为首次从该植物中分离得到。生物学评价表明,化合物4通过抑制lps诱导的BV2小鼠小胶质细胞NO的产生,表现出最强的抗神经炎活性。随后,网络药理学分析表明,化合物4主要通过TNF信号通路缓解神经炎症诱导的认知功能障碍。分子对接研究、分子动力学模拟技术和western blotting分析进一步证实,化合物4可以下调TNF-α和IL-6的表达,抑制炎症相关诱导酶iNOS和COX-2的活性。同时,进一步证明化合物4可以通过TNF途径抑制NF-κB和MAPK信号通路的激活。本文章由计算机程序翻译,如有差异,请以英文原文为准。
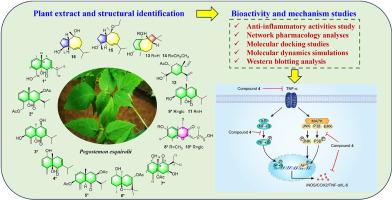
Isolation, characterization, and anti-neuroinflammatory activity of sesquiterpenoids from Pogostemon esquirolii
The phytochemical assessment of Pogostemon esquirolii resulted in the isolation and identification of sixteen sesquiterpenoids, including eudesmane-type (1–6, 9, and 11), bisabolane-type (8 and 10), caryolane-type (13–14), guaiacane-type (15–16), and other types (7 and 12). Of them, compounds 1–10, namely pogostesquines A–J, are ten previously undescribed ones. Compound 7 possessed an unusual backbone with the isopropyl group substituted at C-8 instead of C-7. Their structures were confirmed by extensive spectroscopic analysis, single-crystal X-ray diffraction analysis, and ECD calculations. All the compounds were reported from this plant for the first time. Biological evaluation revealed that compound 4 exhibited the most anti-neuroinflammatory activity by inhibiting NO production in LPS-induced BV2 mouse microglial cells. Subsequently, network pharmacology analyses indicated that compound 4 primarily alleviated neuroinflammation-induced cognitive dysfunction through the TNF signaling pathway. Molecular docking studies, molecular dynamics simulations techniques, and western blotting analysis further clarified that compound 4 could downregulate the expression of TNF-α and IL-6, and inhibit the activity of inflammation-associated inducible enzymes iNOS and COX-2. Concurrently, it was further demonstrated that compound 4 could inhibit the activation of NF-κB and MAPK signaling pathways via the TNF pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


