揭示肺发病机制中的免疫细胞分泌串扰:一种微流控肺芯片方法。
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
肺芯片(Lung-on-a-Chip, LOC)作为一种高度仿生的体外微流控技术,能够精确模拟肺部微环境和生理功能,为肺部疾病的研究提供了重要工具。LOC通过多层通道和生物材料构建肺泡、气道和血管结构,支持多种类型细胞的培养。其微结构设计可以模拟呼吸运动的机械刺激,从而影响细胞行为。免疫细胞及其分泌的细胞因子在维持肺内稳态中起着至关重要的作用,LOC模型为研究免疫细胞的行为特征、信号通路及其与其他细胞的协同相互作用提供了独特的视角。本文旨在利用LOC技术深入探讨免疫细胞及其分泌产物在肺部疾病中的作用机制,为肺部疾病的治疗策略和靶点的开发提供理论基础和创新思路。在未来,LOC技术有望通过个性化医疗、多器官芯片系统和跨学科合作来推进呼吸系统疾病的治疗和药物开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
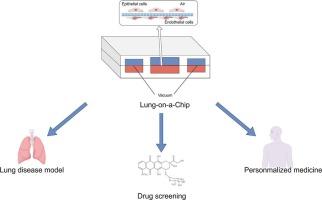
Revealing immune cell secretion crosstalk in pulmonary pathogenesis: A microfluidic lung-on-a-chip approach
As a highly biomimetic in vitro microfluidic technology, the Lung-on-a-Chip (LOC) is capable of precisely simulating the pulmonary microenvironment and physiological functions, thereby providing an essential tool for the study of pulmonary diseases. The LOC constructs alveolar, airway, and vascular structures through multi-layered channels and biomaterials, supporting the culture of various types of cells. Its microstructural design can mimic the mechanical stimuli of respiratory movements, thereby influencing cellular behavior. Immune cells and their secreted cytokines play a crucial role in maintaining pulmonary homeostasis, and the LOC model offers a unique perspective for studying the behavioral characteristics of immune cells, their signaling pathways, and their synergistic interactions with other cells. This review focuses on the in-depth investigation of the mechanisms underlying the role of immune cells and their secretory products in pulmonary diseases using LOC technology, with the aim of providing theoretical foundations and innovative ideas for the development of therapeutic strategies and targets for pulmonary diseases. In the future, LOC technology is expected to advance the treatment and drug development for respiratory diseases through personalized medicine, multi-organ-on-a-chip systems, and interdisciplinary collaborations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


