HPMCAS-L和PVP-VA喷雾干燥分散体利托那韦片的处方优化及溶出/渗透性能比较
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
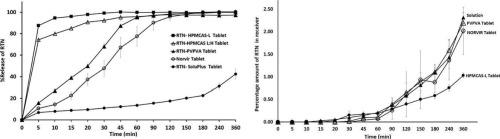
片剂是最优选的口服剂型,因为它具有制造速度快、成本效益高、稳定性好和患者依从性好等优点。非晶固体分散体(ASDs)可以提高难溶性药物的溶解度和生物利用度,但目前缺乏将ASD粉末转化为片剂的策略方法。本研究旨在优化利托那韦- hpmcas - l ASD粉剂的处方和工艺,并比较其与PVP-VA片剂的溶出/渗透性能。以微晶纤维素(MCC)和二碱式磷酸钙(DCP)作为填料,交联纤维素钠(CCS)、交联聚维酮(CP)和淀粉乙醇酸钠(SSG)作为崩解剂。优化考虑了两种SDD粉末水平、两种崩解剂水平和两种喷雾干燥入口温度(70 和140 °C)。进一步的工艺优化测试了不同的压实力通过直接压缩和干燥造粒。利用HPMCAS-L对利托那韦片剂进行优化,确定了以下最佳体外溶出参数:干燥造粒(预压缩14kN +7 kN)、65.5% % SDD中间体、10 % CCS作为崩解剂、24 % MCC作为填充剂和0.5 %硬脂酸镁作为润滑剂。将这些参数应用于由PVP-VA、SoluPlus、HPMCAS-L和HPMCAS-L:H制备的利托那韦ASD片,HPMCAS-L也表现出良好的体外溶出性,与PVPV-VA相似。当进行溶出/渗透评价时,PVP-VA片比HPMCAS-L片表现出更高的利托那韦渗透。PVP-VA片的利托那韦渗透性与诺韦片剂相似,与诺韦粉剂相似。这些结果可能反映了溶解产生的胶体是ASD药物吸收的关键组成部分,甚至超过了有利的溶解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Formulation optimization of ritonavir tablets containing spray dried dispersion from HPMCAS-L or PVP-VA and comparative dissolution/permeation performance
Tablets are the most preferred oral dosage form due to advantages such as high-speed manufacturing, cost-effectiveness, stability, and patient compliance. Amorphous solid dispersions (ASDs) can enhance the solubility and bioavailability of poorly soluble drugs, but a strategic approach to convert ASD powders into tablets is lacking. This study aimed to optimize formulation and process for tablets containing ritonavir-HPMCAS-L ASD powders and compare its dissolution/permeation performance to tablets employing PVP-VA. Microcrystalline cellulose(MCC) and dibasic calcium phosphate(DCP) were evaluated as fillers, while croscarmellose sodium(CCS), crospovidone(CP) and sodium starch glycolate(SSG) served as disintegrants. Optimization considered two SDD powder levels, two disintegrant levels, and two spray-drying inlet temperatures (70 and 140 °C). Further process optimization tested various compaction forces via direct compression and dry granulation. Tablet optimization of ritonavir using HPMCAS-L identified the following optimum parameters, including favorable in vitro dissolution: dry granulation (14kN pre- compression +7 kN compression), 65.5 % SDD intermediate, 10 % CCS as a disintegrant, 24 % MCC as a filler and 0.5 % magnesium stearate as lubricant. Applying these parameters to ritonavir ASD tablets fabricated with PVP-VA, SoluPlus, HPMCAS-L and HPMCAS-L:H resulted in HPMCAS-L also exhibiting favorable in vitro dissolution, like PVPV-VA. When subjected to dissolution/permeation evaluation, tablets with PVP-VA exhibited higher ritonavir permeation than those from HPMCAS-L. Tablets with PVP-VA showed ritonavir permeation like from Norvir tablets, and solution from Norvir powder. These results may reflect the dissolution-resulting colloid as a critical component to overall drug absorption from ASD, beyond even favorable dissolution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


