柔性集成制药固体剂型增材制造过程中API结晶控制。
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
固体剂型(如片剂、胶囊)的传统制造需要多个单元操作和固体处理,已知比处理液体更具挑战性。为了规避这些挑战,本研究探索了液体分配制造口服固体剂型。本发明的增材制造工艺将溶液(原料药、溶剂、聚合物)分配到载体(胶囊)中。在受控的溶剂蒸发后,模型活性药物成分,外消旋莫达非尼(MOD),在聚合物基质(聚乙二醇)内结晶,产生结晶固体分散体。对关键工艺参数(如温度、浓度、蒸发速率、溶剂选择)和关键性能指标进行了评估,以确保稳健的结晶固体分散体制造。耦合结晶和配方工艺在结晶固体分散体中提供所需的MOD多晶形态I,符合商业配方MOD片剂(Provigil®)的质量属性,遵循美国药典方法。此外,开发的工作流程和见解提供了一种适用于其他原料药-聚合物-溶剂体系的可推广的方法,无论是否需要结晶或非晶固体分散体。最终,本研究展示了固体剂型的灵活(添加剂)配方,例如,需要在偏远地区的使用点制造或通过过程强化方法进行个性化医疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
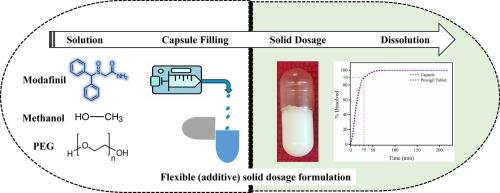
Controlled API crystallization during additive manufacturing of solid dosage form for flexible integrated pharmaceutical manufacturing
Conventional manufacturing of solid dosage forms (e.g., tablets, capsules) demands multiple unit operations and handling of solids, known to be more challenging than handling liquids. To circumvent these challenges, this study explored liquid dispensing to manufacture an oral solid dosage form. The presented additive manufacturing process dispenses a solution (drug substance, solvent, polymer) into a carrier (capsule). Upon controlled solvent evaporation, the model active pharmaceutical ingredient, racemic modafinil (MOD), crystallizes inside a polymer matrix (polyethylene glycol) generating a crystalline solid dispersion. The critical process parameters (e.g., temperature, concentration, evaporation rate, choice of solvent) and key performance metrics were evaluated to ensure robust crystalline solid dispersion manufacturing. The coupled crystallization and formulation process delivers the desired polymorph form I of MOD inside a crystalline solid dispersion that matches the quality attributes of commercially formulated MOD tablets (Provigil®) following US Pharmacopeia methods. Moreover, the developed workflow and insights presented provide a generalizable approach applicable to other drug substance – polymer – solvent systems, independent if crystalline or amorphous solid dispersion is needed. Ultimately, this study demonstrates the flexible (additive) formulation of solid dosage forms, needed for, e.g., point-of-use manufacturing in remote areas or personalized medicine via a process intensification approach.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


