外泌体FGG通过诱导血管生成转移前生态位形成促进结直肠癌肝转移。
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
血管生成是转移前生态位的一个决定性特征,是原发性结直肠癌(CRC)肿瘤转移的必要条件。上皮-间质转化(EMT)也是结直肠癌肿瘤转移进展的关键驱动因素。在这里,我们假设来自结直肠癌细胞的外泌体通过重塑肿瘤微环境促进肝转移。为了验证这一假设,我们从结直肠癌细胞中分离并鉴定了外泌体,并研究了这些外泌体对人脐静脉内皮细胞(HUVECs)的影响。结直肠癌细胞外泌体促进HUVECs的血管化、通透性和迁移。机制上,来自结直肠癌细胞的外泌体传递纤维蛋白原γ (FGG)以发挥其对HUVECs的作用。FGG下调CRC细胞中VE-cadherin和E-cadherin水平,上调N-cadherin和vimentin水平,从而增强内皮通透性,促进EMT。体内实验表明,FGG下调结直肠癌组织中的VE-cadherin,上调肝组织中的CD31,最终导致结直肠癌肝转移小鼠模型中转移性肝结节数量增加。综上所述,FGG通过外泌体介导的机制调节关键的血管生成、粘附和间质标志物,促进结直肠癌肝转移,从而增强血管生成、血管通透性和EMT诱导。这些发现为结直肠癌肝转移的机制和治疗策略提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
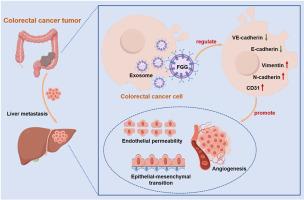
Exosomal FGG promotes colorectal cancer liver metastases through inducing angiogenic pre-metastatic niche formation
Angiogenesis is a defining feature of a pre-metastatic niche and is essential for primary colorectal cancer (CRC) tumor metastasis. Epithelial-mesenchymal transition (EMT) also serves as a critical driver of CRC tumor metastatic progression. Here, we hypothesized that exosomes from CRC cells promoted liver metastasis by remodeling tumor microenvironment. To verify this hypothesis, exosomes from CRC cells were isolated and identified, and the effects of these exosomes on human umbilical vein endothelial cells (HUVECs) were investigated. Exosomes from CRC cells promoted vascularization, permeability and migration of HUVECs. Mechanistically, exosomes derived from CRC cells delivered Fibrinogen gamma (FGG) to exert their effects on HUVECs. Furthermore, FGG downregulated the levels of VE-cadherin and E-cadherin in CRC cells, while upregulating N-cadherin and vimentin levels, thereby enhancing endothelial permeability and promoting EMT. In vivo experiments demonstrated that FGG downregulated VE-cadherin in CRC tissues and upregulated CD31 in liver tissues, ultimately leading to an increased number of metastatic liver nodules in a mouse model of CRC liver metastasis. In conclusion, FGG facilitates CRC liver metastasis by regulating key angiogenic, adhesion and mesenchymal markers via exosome-mediated mechanisms, resulting enhanced angiogenesis, vascular permeability, and EMT induction. These findings offer new insights into the mechanisms and treatment strategies of CRC liver metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


